-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
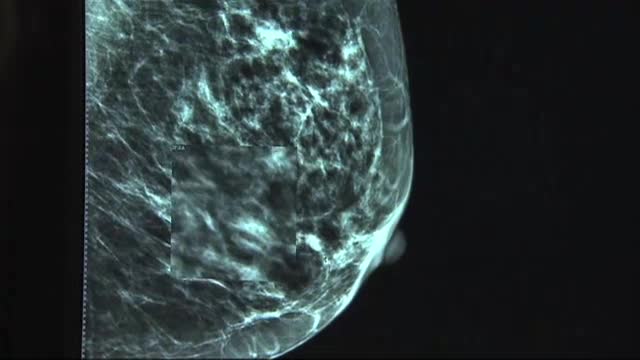
Gene Test May Help a few Patients Skip Chemotherapy
The New York Times reports that according to Dr. Elyce H. Cardonick, a maternal-fetal specialist at Cooper Medical School of Rowan University in Camden, N.J., “The main message of this study is that termination of pregnancy is not necessarily warranted, and that early preterm delivery to be able to do cancer treatment isn’t warranted, either”.
Advertisement
“Where this will help us make a decision for the patients, a few patients may not need chemotherapy and end up getting it if you did not have this test”, Shinwari says.
The findings were published earlier today, September 28, 2015, in the New England Journal of Medicine. In the U.S., breast cancer is the second most common cancer in women after skin cancer, affecting about 230,000 new patients annually. This secondary option consists of surgery and years of taking a hormone-blocking drug. With half a million patients tested in more than 90 countries, the Oncotype DX tests have redefined personalized medicine by making genomics a critical part of cancer diagnosis and treatment. The test that measures these factors to determine whether women are low-risk, high-risk, or somewhere in between-called Oncotype DX-has been on the market since 2004. About 16 percent of the women scored low on the test and thus, received only anti-hormone therapy, no chemotherapy. Researchers observed no difference whether women underwent chemotherapy (100 vs. 99.5), radiation therapy (102 vs. 105), surgery alone (111 vs. 102) or no treatment (105 vs. 97.5).
The European study emphasized, however, that the women in the study did not receive treatment during the first 12 to 14 weeks of their pregnancy when the risk of birth defects is greatest. A 5-year follow-up revealed that the survival rate was at 98 percent, with less than a 2 percent chance that the cancer had spread in the body.
Breast cancer is mainly a disease of old age and 40% of new breast cancer cases occur in patients aged 65 and older.
“The compelling results seen in this global study provide unequivocal evidence supporting the clinical utility of Oncotype DX to risk-stratify patients with early-stage breast cancer, and indicate that the findings are generalizable to everyday clinical practice”, Dr. Sparano said.
The use of hormone therapy and chemotherapy (systemic therapy) in Belgium, Ireland, The Netherlands, Portugal and Poland, varied substantially depending on the stage of the breast cancer.
“Patients love the idea of a test” to help reduce uncertainty about treatment, she said. Also, they are urged to have chemo which kills any stray cancer cells if spread beyond the breast. Doctors tend to rely on the assay when they want more information before deciding whether to recommend chemotherapy to their patients. “I’ve had chemotherapy. It’s not pretty”.
Advertisement
The tests now will cost $4,175 but it is covered by many insurers.