-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
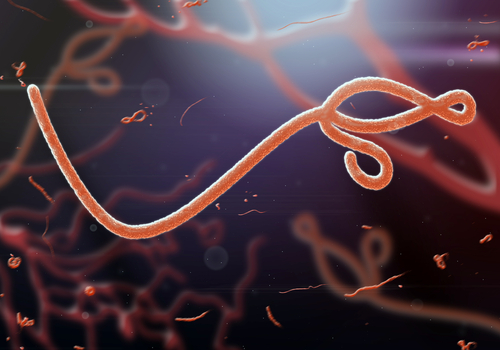
Ebola drug trial stops adding patients | SBS News
Shares of drugmaker Tekmira slumped Friday after the company said it stopped enrolling new patients in a trial of a treatment for a strain of Ebola.
Advertisement
The Canadian company said the mid-stage study reached a statistical futility boundary, meaning it’s unlikely the trial will show the drug worked.
“The endpoint indicated that continuing enrollment was not likely to demonstrate an overall therapeutic benefit”, the news release stated.
Thomas Geisbert, a specialist in infectious diseases at the University of Texas Medical Branch, said the end of the trial not at all means that the drug is not effective; it’s just shows complexities associated with testing drugs on real patients during epidemics.
Data analysis is ongoing and full results will be made available as soon as possible, the firm said.
The medication was created to treat the Ebola-Guinea strain, also called Ebola virus Makona, responsible for the current outbreak in West Africa. The Phase II single arm trial called RAPIDE (Rapid Assessment of Potential Interventions & Drugs for Ebola) is open-label with a concurrent observational study of Ebola virus disease in Sierra Leone. More than 11,000 people have died since that outbreak began in December 2013.
Horby is with the University of Oxford, which was conducting the trial. Tekmira expects results from that study in the second half of this year.
Advertisement