-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
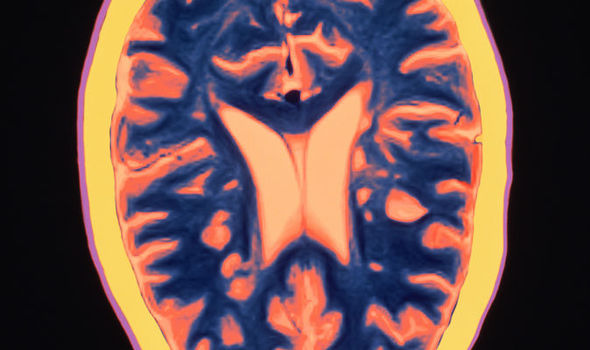
First drug ‘slows decline’ in progressive MS
In the two relapsing Mississippi trials, the new drug reduced the annualized relapse rate by 46 and 47 percent compared to Merck KGaA’s Rebif, while the rate of confirmed disability progression (CDP) was 37 and 43 percent lower. Multiple sclerosis, which affects about 2.3 million people worldwide, is a chronic and incurable disease that destroys the nerves and robs patients of their ability to control their movements. While acute relapses typically happen perhaps once a year in the relapsing form of the disease, magnetic resonance imaging studies have shown that bursts of inflammatory attacks on myelin are much more frequent, he said.
Advertisement
‘People with primary progressive Mississippi and clinicians alike have been eagerly waiting for an effective treatment to slow the path of relentless deterioration’.
Mississippi patients and their advocates welcomed Thursday’s announcement, especially for those with the progressive form who now are limited to drugs that reduce symptoms but don’t address the mechanisms of the disease. A result of the body’s own immune system’s attacking myelin sheaths that coat nerve fibers, the disease typically strikes between the ages of 20 and 40, and affects women more commonly than men.
Pharmaceutical company Roche has a much-hyped multiple sclerosis drug called ocrelizumab which has, apparently lived up to the hype.
An experimental multiple sclerosis medicine from Roche AG is offering hope for the first time to a group of patients whose symptoms don’t let up after diagnosis.
The watchdog, the National Institute for Health and Care Excellence (NICE), will then assess whether it is cost effective for use on the NHS.
Described as a “game changer” by scientists, ocrelizumab is the first ever drug to be shown to effectively treat the 10 per cent of Mississippi patients with a form called primary progressive MS.
“We want to make the medicine available for patients as soon as possible”, Fontoura said.
Former British jazz pianist of the year Craig Milverton has been taking the drug since 2012.
Dr Klaus Schmierer of Queen Mary University of London, said: ‘This is great news for everybody affected by Mississippi, and society as a whole.
Results of the separate study of patients with the progressive form of Mississippi won’t be released until Saturday, but Hauser said they are equally promising.
“It’s the most promising I’ve felt in a very long time”, she said of the potential of the new drug.
Roche hasn’t yet published full results from the studies, which were sponsored by Roche and its Genentech unit.
“So it has been unbelievable for me because I really thought my career was over”.
Advertisement
“We hope such an encouraging outcome will stimulate further progress in beating this disease”.