-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
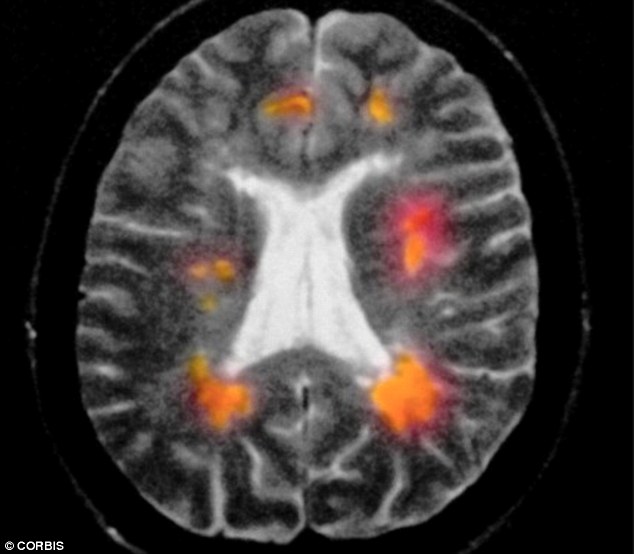
Roche Reports Positive Late-Stage Trial Results for MS Drug
Swiss drugmaker Roche says its experimental medicine for multiple sclerosis, ocrelizumab, performed better in a late-stage clinical trial than a commonly used therapy for the most prevalent form of MS. Mississippi affects women more than man, according to Centers for the Disease and Control Prevention (CDC). “Ocrelizumab is the first investigational medicine to significantly reduce disability progression in people with relapsing MS and people with primary progressive MS – a form of MS with no approved treatments”.
Advertisement
Given that the drug is created to selectively target CD20-positive B cells, Roche said the fact that efficacy was shown across both forms of Mississippi “validates the hypothesis that B cells are central to the underlying biology of the disease”.
“This is potentially a big deal for our patients”, said Stephen Hauser, chief of neurology at the University of California San Francisco School of Medicine and a leader of the two studies in relapsing multiple sclerosis, The Wall Street Journal reported.
The manufacturers, Roche, are now expected to apply for a medicines licence from the regulatory authorities.
Genentech plans to submit data for the treatment to the U.S. Food and Drug Administration early next year for approval.
Multiple sclerosis is a chronic disease that affects an estimated 2.3 million people around the world, according to the company.
Drug safety is critical in Mississippi, since the disease is caused by abnormal immune system attacks on the protective sheath surrounding nerve cells and treatments need to adjust the body’s response, which can lead to problems such as infections.
Dr Klaus Schmierer, a consultant neurologist at The Royal London Hospital, said: “People with primary progressive Mississippi and clinicians alike have been eagerly waiting for an effective treatment to slow the path of relentless deterioration”.
In recent years a host of new drugs have reached the market that have been especially effective against the relapsing form of the disease, and slowed development of progressive multiple sclerosis, Dr. Hauser said. “The community now believes, for many people with Mississippi, more highly effective therapies earlier in the disease is likely to be very helpful over the long run”, he said.
This new treatment represents a great advance for those who suffer from the disease. In the relapsing disease studies, the drug reduced such bursts by at least 94%, he said. Her concern is that her symptoms, which continue to plague her, will worsen if and when she has a second child.
Advertisement
“So far only the top line results from this trial have been announced, so we look forward to seeing the full details with great anticipation”.