-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
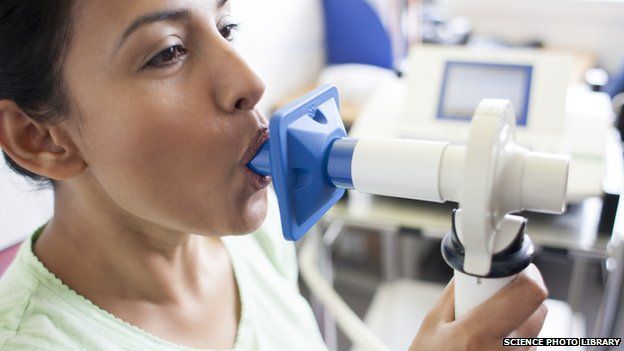
Therapy to improve lung function in cystic fibrosis patients
The patients received either monthly doses of the gene therapy or a placebo for 1 year.
Advertisement
After experimenting the new treatment on 62 patients, researchers observed improvement in the lung functions of all study subjects.
The experiment took place at two hospitals, the Royal Brompton, in London and the Western General Hospital in Edinburgh between June 2012 and 2013.
Prof Stuart Elborn, of Queen’s University in Belfast, said the results were “encouraging” but the therapy had been no more effective than some of the drugs currently available.
The trial – run by the United Kingdom Cystic Fibrosis Gene Therapy Consortium – now needs to be followed by further studies to assess the optimum dose and treatment schedule, they add.
The current trial was launched in 2012 and funded by a partnership between the Medical Research Council (MRC) and the National Institute for Health Research (NIHR). The research was published online Thursday in the journal, Lancet Respiratory Medicine.
Cystic fibrosis is a rare, inherited respiratory disease that is caused by mutations in a gene called cystic fibrosis transmembrane conductance regulator, (CFTR). The lung function of patients improved after taking the gene-altering medicine.
Although the benefits observed were the result of a stabilisation of lung function rather than an actual improvement, the results of this study are still encouraging. He added: “We are actively pursuing further studies of non-viral gene therapy looking at different doses and combinations of other treatments, and more efficient vectors”. The programme of work came about through the generosity of the CF community via the CF Trust, whilst the trial exemplifies how effectively the MRC and NIHR, working in partnership, are able to move laboratory science into patient benefit.
Ireland has a particularly strong attachment with the treatment and search for a solution to end CF as the country is statistically the most likely to receive the faulty gene with an average of one person in 19 carrying it.
“If the bigger study shows big benefits then it’s feasible that we’ll be able to offer this treatment to patients by the end of the decade”.
Imperial Innovations, Imperial’s technology commercialisation company, is acting as the lead technology transfer office for the consortium.
Patients were randomized into a number of stratified subgroups, but the authors attributed any treatment effect to a greater decline in FEV1 from the placebo group as opposed to greater improvement from pGM169/GL67A. But all attempts so far have been thwarted by the body’s protective mechanisms for clearing unwanted material out of the lungs and fighting off foreign invaders such as viruses, which are used to ferry corrective DNA into cells. First clinical trials are expected to start in 2016.
Advertisement
She says her team has some preliminary data showing that techniques like this could work, potentially allowing us to take advantage of the lungs’ position at the centre of our circulatory system for their delivery. Alton said that while a few children reported feeling better, others didn’t feel any difference. Over the course of a year, those who benefited from the treatment exhibited 3.7% better lung function compared to those who were given placebo medication only.