-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
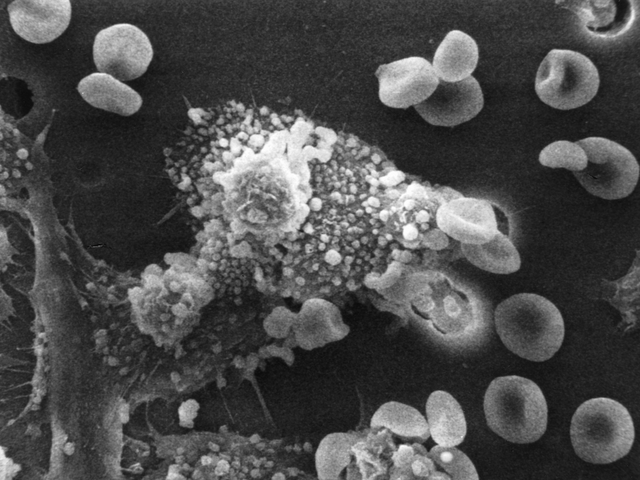
FDA expands use of melanoma drug
This program provides earlier patient access to promising new drugs while the company conducts confirmatory clinical trials. “The approval of Yervoy for the treatment of adjuvant melanoma underscores our scientific leadership in Immuno-Oncology, with a commitment to further developing our I-O agents – Yervoy and Opdivo – across multiple tumor types and at many stages of disease”.
Advertisement
Patients with stage 3 melanoma can now receive the intravenous drug as an adjunct therapy. Clinical trials showed that Imlygic was able to shrink a tumor significantly more than control patients who only received the GM-CSF protein therapy. It’s injected directly into melanoma lesions, where it reproduces inside cancer cells and is created to rupture and kill those cells, the agency said.
IMLYGIC is a version of the herpes simplex virus that has been genetically modified to attenuate the virus, increase selectivity for cancer cells, and secrete cytokine granulocyte-macrophage colony-stimulating factor (GM-CSF). While 49% of patients on ipilimumab saw a recurrence by an average of 26 months post-treatment, 62% of those on placebo had their cancer return within 17 months.
The investigators found that common side effects of the drug were rash, diarrhea, fatigue, itching, headache, weight loss and nausea. “Not only does this combination immunotherapy demonstrate an impressive, durable response in patients with metastatic melanoma, but the approval also illustrates the potential advantage of combining immunotherapy agents offering previously unavailable options to cancer patients”. It is the first-ever viral oncolytic. This collaborative work was enabled by Amgen, manufacturer of the T-VEC virus and will be marketed under the name IMLYGICTM.
Advertisement
The approval was applauded by the Melanoma Research Foundation in a statement from Tim Turnham, the organization’s Executive Director: “The approval marks a turning point in melanoma treatment”. After surgery, Yervoy can be used to reduce the risk of recurring melanoma. The HCI Cancer Learning Center for patient and public education contains one of the nation’s largest collections of cancer-related publications. The institute is named after Jon M. Huntsman, a Utah philanthropist, industrialist, and cancer survivor.