-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
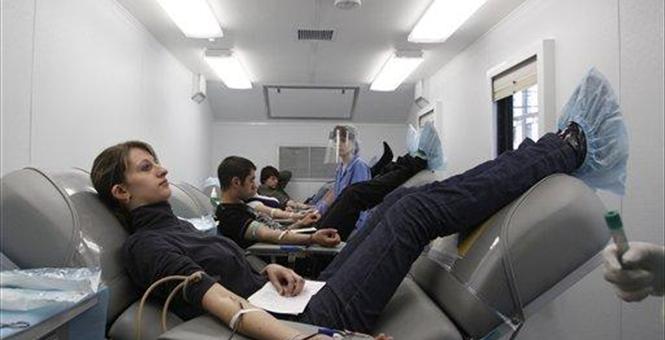
US FDA overturns 30-year ban on blood donations by gay men
The nation’s three-decade-old ban on blood donations from gay and bisexual men was formally lifted Monday, but major restrictions will continue to limit who can donate.
Advertisement
Peter Marks, M.D., Ph.D., deputy director of the FDA’s Center for Biologics Evaluation and Research, defended the one-year decision in the FDA announcement, saying the agency had “rigorously examined several alternative options” but that the deferral window was “supported by the best available scientific evidence, at this point in time, relevant to the US population”. The FDA examined a variety of recent studies, epidemiologic data, and shared experiences from other countries that have made recent MSM deferral policy changes.
Jason Cianciotto, GMHC’s public policy director, stands with a map showing worldwide blood donation guidelines for men who have sex with men, in the organization’s offices in NY on Tuesday, Sept. 10, 2013. “While it’s a step in the right direction toward an ideal policy that reflects the best scientific research, it still falls far short of a fully acceptable solution because it continues to stigmatize gay and bisexual men”.
The agency said it has also put in place a safety monitoring system for the blood supply which it expects to provide “critical information” to help inform future FDA blood donor policies.
A leading HIV/AIDS advocacy group, the Gay Men’s Health Crisis, has said the government’s decision to keep the one-year ban – which they say is de facto a lifetime ban, remains “offensive and harmful”.
This will bring the U.S. in line with countries like the United Kingdom and Australia, where gay and bisexual men can donate blood if they have not had sex in the past twelve months.
Quigley said in a statement the LGBT community has enjoyed significant advancements in recent years, but the change in blood donation policy “does not keep up with that same progress”.
For decades, gay men in America have been prohibited from donating blood. Most said it shouldn’t matter if prospective male donors had had sex with another man.
On December 21, 2015, the FDA revealed a change in the deferral period in the latest guidance.
Earnest maintained the president has “a very strong record” on LGBT rights, but said other factors are a consideration.
Advertisement
“We strongly encourage the FDA to move toward a deferral based upon individual risk assessment”, Yeszak said.