-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
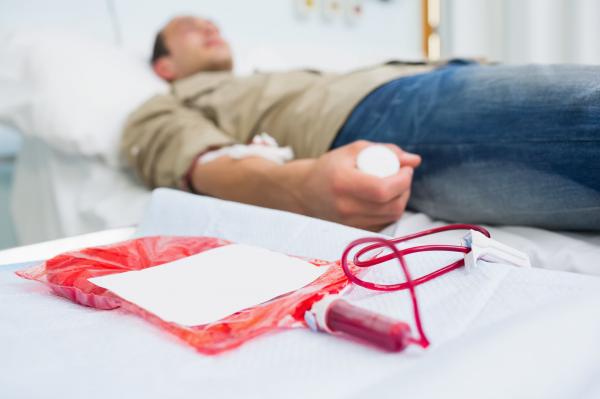
FDA Lifts Ban On Blood Donations By Gay Men
The lifetime ban, which had been in effect for 32 years, will be substituted by a new policy that will allow blood donations from gay men, provided they have not had sexual relations with another man for at least a year, the FDA announced Monday.
Advertisement
Groups that handle blood donations lobbied for the change, calling the ban on gay donors “medically unwarranted”.
The move was met with criticism from gay rights groups who-along with the medical community-have been pushing against the policy for years.
“It continues to stigmatize gay and bisexual men”, said Mr Stacy, a spokesman the Human Rights Campaign. “The reality is now that the FDA has affirmed and agrees that gay men can now donate blood”.
The change brings the United States into line with New Zealand, which reduced its deferral from five years to 12 months last December, after having shifted from a 10-year ban in 2009.
According to the U.S. Food and Drug Administration, the federal body that removed the ban, its decision to reverse its decades-long ban was based on examination of the latest science showing that a blanket prohibition on gay donors is not necessary to prevent HIV transmission.
Dr. Peter Marks, deputy director of the FDA’s Center for Biologics Evaluation and Research, said that the requirement for yearlong celibacy for MSM is supported by scientific evidence relevant to the USA population.
“The FDA’s responsibility is to maintain a high level of blood product safety for people whose lives depend on it. We have taken great care to ensure this policy revision is backed by sound science and continues to protect our blood supply.”
The Whitman-Walker Clinic has called for a deferral period of no more than 30 days, “given that with current testing technology an HIV infection can be detected in donated blood within several weeks of exposure”, he said.
The non-profit Whitman-Walker center has served D.C. residents since 1978, one year after the start of the AIDS epidemic in the U.S. While they offer primary health services, a large portion of their mission is providing HIV education, prevention, and testing services. About 15.7 million blood donations are collected in the USA each year.
Advertisement
“We had people come and donate blood on our behalf, and we worked with the Red Cross”, says Michael Quint of Portland, who organized a local Gay Blood Drive Event a year ago as part of a national effort to push the FDA to change its policy. And, according to the FDA, studies found no change in risk to the blood supply.