-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
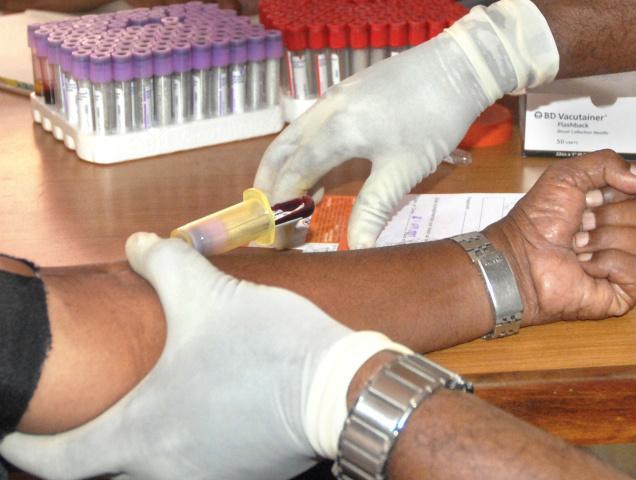
French Prosecutors investigating Botched Clinical Drug Trial Which kills One in France
A man who was left brain-dead during a drugs trial in France has died, while five others remain in hospital with grave fears for their recovery.
Advertisement
The trial was in phase 1, in which healthy volunteers take the medication so its safety and pharmacological profile can be assessed.
Of the 90 participants, 84 have been tested and 5 are in stable condition in a hospital in the city of Rennes.
In the trial, run by the private research company Biotrial, the 6 men starting taking the investigational drug January 7, in varying doses.
Ninety volunteers took the drug, which was manufactured by the Portuguese company Bial, according to the BBC. They entered the hospital in critical neurological condition.
On Friday, the chief neuroscientist at the hospital, Gilles Edan, said there was no known antidote to the drug. The Portuguese drug agency, Infarmed, said that six people have been hospitalised.
The firm said that a total of 108 people have received the medicine – which acts on the endocannabinoid system to target pain – as part of the study, “without any moderate of serious adverse reaction”.
The controversial drug was also meant to ease mood and anxiety troubles.
French health authorities said three of the hospitalised volunteers face possible brain damage and an investigation is under way into what French health minister Marisol Touraine called “an accident of exceptional gravity”.
One suffered brain death and died on Sunday. Four have “neurological problems” and one has no symptoms but is still being monitored.
He was among six male volunteers between 28 and 49 hospitalized last week after volunteering to take the drug.
The big question in France: what went terribly wrong? All of them were part of a drug test of Bial drug.
Biotrial, which has been carrying out drug trials since 1989, said Sunday that the situation is “even more upsetting given that there is as yet no explanation”.
According to reports, the lab Biotrial was in Phase 1 of the trial, meaning that it was the first time it had been tested on humans.
Advertisement
French authorities say one man in comatose, classified as “brain dead”.