-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
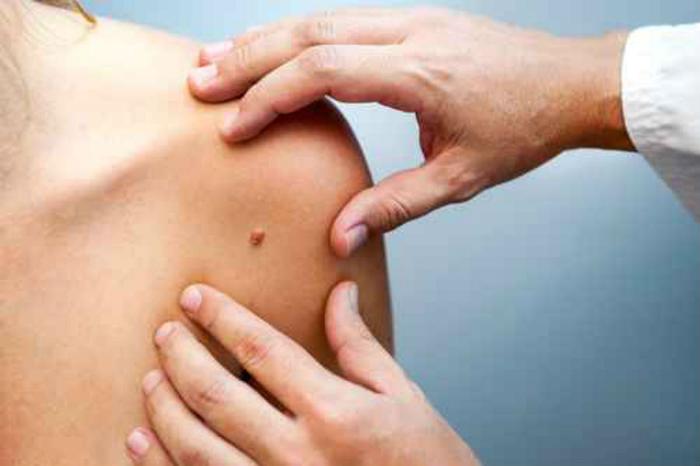
FDA Gives a Go-Ahead to Daily Pill for Common Skin Cancer
The introduction of Erivedge marked the first FDA-approved drug to treat locally advanced and metastatic basal cell carcinoma.
Advertisement
The drug, Odomzo, take a encased caution to really point it out to negative aspects that it can kill or major childbirth blemishes within a establishing unborn child in the event that applied to any expecting a baby female.
Basal cell carcinoma is the abnormal, uncontrolled growth that happens in the skin’s basal cells present in the deepest layer of the epidermis.
Basal cell skin cancers usually result from regular exposure to the sun and other forms of ultraviolet radiation, the FDA says. This type of cancer is responsible for over 80% of non-melanoma skin cancers. With the nose being the most typical site, they occur most frequently about the mind and throat.
Locally advanced basal cell skin cancer refers to basal cancers that have not spread to other parts of the body, but can’t be cured with local treatments, such as surgery and radiation.
Approval was based on clinical trials showing an overall response rate – the percentage of patients who experienced partial shrinkage or complete disappearance of their tumour – of 58% in those given the drug. It functions by curbing the molecular pathway, which can be not inactive in basal cell malignancies.
The drug is an oral, selective smoothened inhibitor, which handles hedgehog signalling pathway.
The outcome showed that 58PERCENT of clients who required 200 mg of Odomzo each day found their cancers reduce or disappear.
This drug should not be taken by women who are pregnant.
Our increasing understanding of molecular pathways involved in cancer has led to approvals of many oncology drugs in difficult-to-treat diseases for which few therapeutic options previously existed, said Richard Pazdur, M.D., director of the Office of Hematology and Oncology Products in the FDAs Center for Drug Evaluation and Research. This effect lasted for between 1.9 and 18.6 months, with around half of the responding patients experiencing it for at least 6 months. The response rates were similar in patients who received vismodegib 800 mg daily; however, adverse effects were more common at that dose. Genentech of Bay Area, Florida markets the medicine.
At the lower dose, the most common side effects were muscle spasms, hair loss, taste problems, fatigue, nausea, musculoskeletal pain, diarrhea, decreased weight and decreased appetite.
Advertisement
The drug can also cause serious musculoskeletal side effects; the FDA also mention rare reports of muscle tissue breakdown.