-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
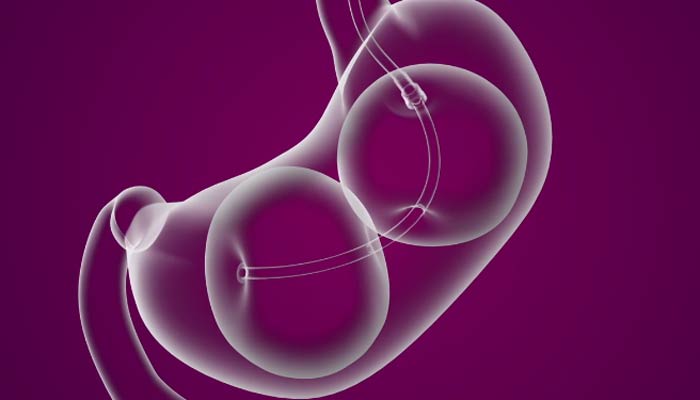
FDA OKs Nonsurgical Device for Treating Obesity
The ReShape Dual balloon is inserted into the stomach through the mouth of the patient and then filled with a sterile solution. The balloons take up space in the stomach, which presumably makes people feel fuller faster, the FDA said in its announcement Tuesday.
Advertisement
Dr. Ninh Nguyen, past president of the American Society for Metabolic and Bariatric Surgery, said in a company release that the procedure “opens up a new opportunity” for patients who do not qualify for weight loss surgery.
The device does not change or alter the stomach’s natural anatomy.
The outpatient procedure takes less than 30 minutes, according to the agency, which noted that the temporary device should be removed 6 months after insertion. “Positive data from the rigorously designed pivotal study, coupled with commercial experience in Europe, demonstrate that ReShape can provide an important new non-surgical option to help patients in the U.S. lose weight and give them the tools needed to keep it off”.
The ReShape Dual Balloon is indicated for weight reduction in obese adult patients with a body mass index (BMI) of 30 to 40 kg/m. The device is limited to patients with one or more obesity-related conditions such as high blood pressure, high cholesterol, and diabetes. It is also the condition that they were unable to reduce weight through other measures like diet and exercise alone.
A similar device was approved by the FDA before but was recalled in 1992 because of safety issues by which, the device can burst and clog peripheral arteries.
“Anyone who has struggled with diet and exercise to lose weight knows it can be a very frustrating cycle”.
It is inserted in the stomach endoscopically and filled with saline and remains in the body for six months.
It is estimated that almost one-third of the American population is obese.
A problem with many weight loss programs is that not uncommonly, after completing a weight loss program, the individual gains back the weight. But six months after the devices’ removal, patients in the balloon group regained about one-third of the weight they had lost.
Potential side effects for the procedure, the FDA warned, include headache, muscle pain, and nausea from the sedation and procedure; in rare cases, severe allergic reaction, heart attack, esophageal tear, infection, and breathing difficulties can occur. The device may also cause vomiting, abdominal pain, and gastric ulcers when the device has been placed in the stomach.
Advertisement
This device should not be used in patients who have had previous gastrointestinal or bariatric surgery or who have been diagnosed with inflammatory intestinal or bowel disease, large hiatal hernia, symptoms of delayed gastric emptying or active H. Pylori infection, nor is it for those who use aspirin daily or who are pregnant.