-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
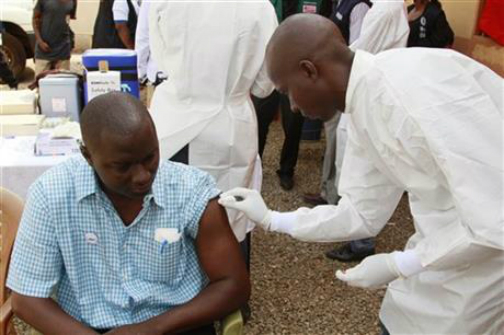
Experimental Ebola vaccine that was tested in Knoxville could stop virus in
The success of the Guinea trial is a big relief for researchers, many of whom feared a sharp decline in cases this year would scupper their hopes of proving a vaccine could work. There have been no cases of Ebola in Knoxville.
Advertisement
The Ebola vaccine from a trial in Guinea needs to be kept at a temperature of minus 60 degrees Celsius, the World Health Organization says. So far, more than 4,000 adults who had close contact with about 100 Ebola patients – including relatives, neighbors and co-workers – have voluntarily taken part in the trial.
The Centers for Disease Control and Prevention and the government of Sierra Leone are also now running vaccine trials on health and frontline workers, who are exposed to Ebola.
Added Peter Smith of the London School of Hygiene & Tropical Medicine, the results were “very exciting and suggest that the Ebola vaccine tested may be highly effective in protecting against Ebola disease among those in the immediate vicinity of an Ebola case”.
“If these results are confirmed as the trial continues, the ring vaccination strategy may be important not only in contributing to ending the current epidemic, but will also be deployable to contain rapidly any future outbreaks of Ebola disease”.
Researchers will begin another trial soon on another vaccination for Ebola. She on July 31, 2015 said Ebola vaccine was near.
The study of the Merck vaccine was led by Ana Maria Henao-Restrepo of the World Health Organization (WHO) in Geneva, together with colleagues at the Norwegian Institute of Public Health in Oslo, the Guinean Ministry of Health, and others.
Some of the researchers involved with the study said that “they hadn’t expected such clear results”, explains Sheri Fink, a medical journalist who has been writing about Ebola for the New York Times.
A second comparison group of 3528 people received the vaccine three weeks after potential exposure. The private-public partnership, which often buys immunizations for poor countries, said Friday that it “stands ready to support the implementation of a WHO-recommended Ebola vaccine”. In a small trial, an experimental vaccine protected 100 percent of the people at high risk for the virus. “A significant obstacle to an effective response has been the inadequate engagement with affected communities and families”, the report says.
A number of other groups are at an earlier stage of research, including a team from the University of Texas working on an inhaled vaccine.
There have been a total of 27,748 cases of Ebola in Guinea, Liberia and Sierra Leone, with 11,279 reported deaths.
“This (vaccine) could be the key that we’ve been missing to end the outbreak”, Neuman said.
Advertisement
“When there is an urgency to save lives, research and development can be fast-tracked and made to work for the common good”, says Dr. Marie-Paule Kieny, WHO assistant-director general.