-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
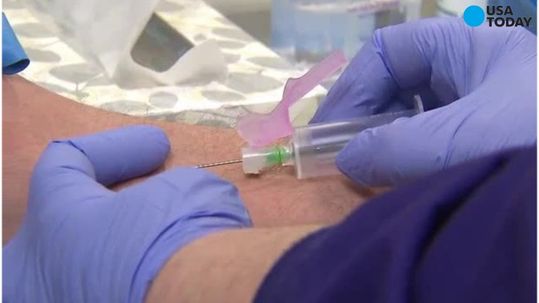
Theranos sued over blood tests, in proposed class action
The company told federal regulators with the U.S. Center for Medicare and Medicaid Services about their decision to void the test results for the two year period. A single Theranos customer is bringing this lawsuit on behalf of himself and two other potential classes of consumers – people who bought Theranos tests in Arizona as well as nationwide.
Advertisement
Now, adding to its woes, the company has been accused in a lawsuit of misleading consumers about the accuracy of its test results and the reliability of its technology. The Wall Street Journal reported Walgreens did not verify whether or not the technology actually functioned before the pharmaceutical firm inked a deal with the diagnostic start-up in 2013 that entailed opening Theranos testing clinics in dozens of Walgreens franchises. These wellness centers were closed in California last January in light of an alarming regulatory inspection report. Now, she counts billionaire Larry Ellison as an investor in her company, Theranos.
“As a result (of the newly voided results), tens of thousands of patients may have been given incorrect blood-test results, been subject to unnecessary or potentially harmful treatments, and/or been denied the opportunity to seek treatment for a treatable condition”, reads the complaint, which was provided to USA TODAY.
The suit contends that Theranos falsely marketed its fingerprick testing method, which promised to disrupt the $75 billion blood testing industry by asking patients to provide mere drops, and not vials, of blood to be processed by its innovative if secretive Edison machine.
From media coverage and reports from federal regulators, it is now clear that Edison devices were only ever used to run 12 tests and that they were inaccurate and unreliable.
An anonymous Arizona plaintiff, identified only as “M.P.B.”, filed the lawsuit in California on Wednesday. “The company will vigorously defend itself against these claims”, she said.
The drugstore giant agreed to put Theranos blood-testing centers in thousands of its drugstores across the USA without ever fully validating the startup’s technology or its capabilities, The Wall Street Journal reports. Since issues with Theranos’ testing have been raised, Walgreens has distanced itself from the company and has discussed terminating its relationship. Theranos last week notified United States federal health regulators that it voided results from its Edison blood-testing devices for two years, the Wall Street Journal reported, citing a person familiar with the matter.
Advertisement
This is just the latest controversy surrounding the company, which was founded in 2003 by 19-year-old Elizabeth Holmes.