-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
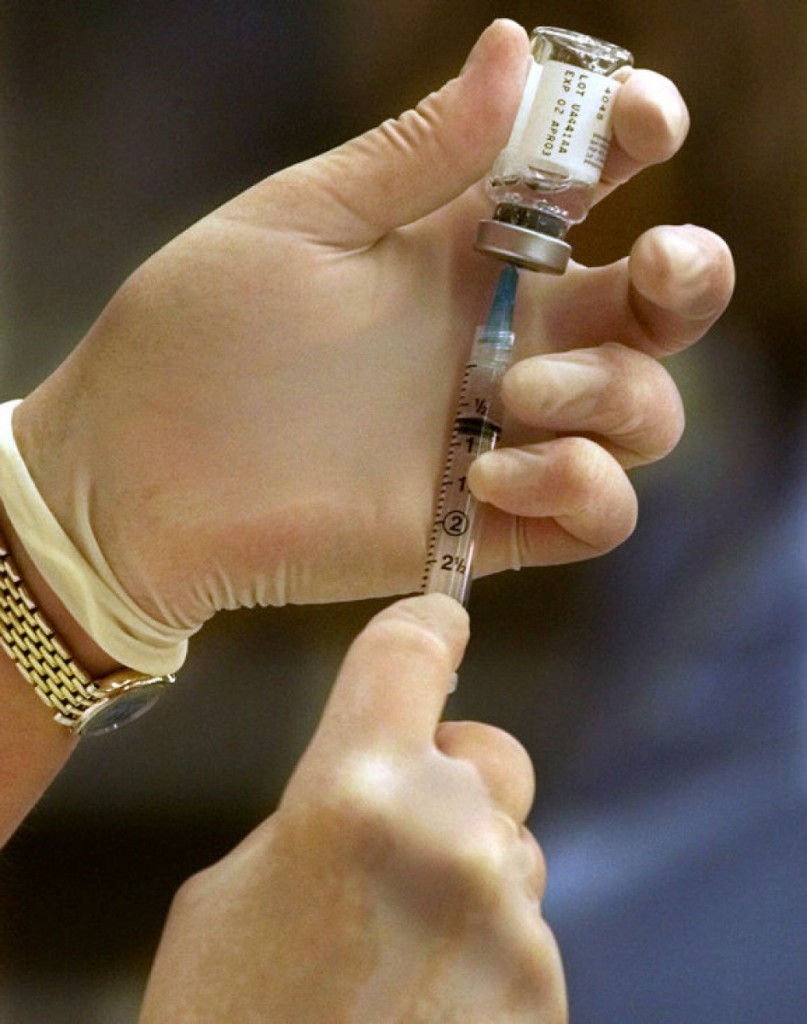
Global vaccine fund could prevent deadly disease outbreak
So far, it has killed 11,000 people out of the 28,000 infected in only the three countries and cases are still being reported.
Advertisement
How did the vaccine come about?
This is the first time an experimental Ebola vaccine has been shown to prevent people from becoming infected with the deadly disease.
It should be mentioned that people who were immediately administered the vaccine have not contracted the virus.
“The hope was a vaccine might be developed in sufficient time to have an impact to help control and maybe help eliminate this Ebola outbreak if it were to persist”, said Mark Feinberg, chief public-health and scientific officer for Merck, according to the Wall Street Journal. Essentially, all of the family, friends, and neighbors of a confirmed Ebola case are vaccinated, generating a protective ring around the case to keep the disease from spreading to people beyond the ring.
100% protection sounds too good to be true.
.
“People fear Ebola because there are no approved treatments or vaccines for infection, and 50-90 percent of the people who get it die”, said Dr. Gunn-Dye.
For the trials, half of the participants were vaccinated three weeks after identification of an infected patient. “The thousands of volunteers from Conakry and other areas of Lower Guinea, but also the many Guinean doctors, data managers and community mobilizers have contributed to finding a line of defense against a awful disease”. But there is already excitement about the vaccine. While the vaccine may prove to be a little less than 100 per cent effective in the long run, this is still a remarkable success story. The group included Professor Donald A. Henderson of John Hopkins University, who led the WHO smallpox eradication effort by using the ring vaccination approach. But this is extremely encouraging news that may herald the beginning of the end of the kind of outbreaks we’ve seen over the last few decades. “Will it work at six months?”
This is one of two vaccines being tested at present in the Ebola-impacted states.
“To be able to conduct a study like this and find these results when case numbers were plummeting…”
Health officials involved in the research have recommended distributing the vaccine where the disease is still active – namely, in Guinea and Sierra Leone.
As of July 2015, clinical trials were ongoing. Though, with the needed support, they would make it so that vaccines would be available for emergency use. That means that outbreaks tend to be over before trials can even begin. The researchers thought it would take about 10 days, and designed the trial to start assessing the vaccine’s efficacy after that point. The urgency of tackling Ebola changed all that. “The study did provide strong evidence supporting vaccine efficacy”. The rVSV-ZEBOV trial is one of several that came about as a result. Also the NewLink Genetics USA participated in the development of the vaccine and it is manufactured by Merck Vaccines. “I think is really a major public health achievement”, said Michael Osterholm, director of the Center for Infectious Diseases Research and Policy at the University of Minnesota. Pathogens considered priority health threats include Marburg virus, which is in the same family as Ebola, and the viruses that cause Middle East respiratory syndrome (MERS), Lassa fever and chikungunya. Because she studies the nuts and bolts of Ebola virus infection, it’s possible that she will learn lessons and develop strategies that are broadly applicable to the fight against other viruses.
Advertisement
In fact, the cost that the global vaccine development involves is the biggest issue, because there are very few companies who want to invest in it. And there seems to be support for change at the highest level.