-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
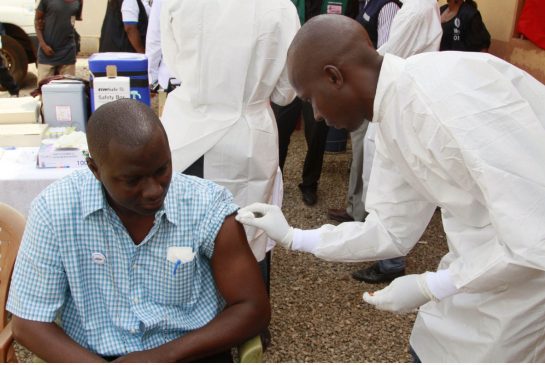
Experimental Ebola vaccine could stop virus in West Africa, study says
In spite of this, an experiment vaccine was tested on thousands of people in Guinea exposed to Ebola seems to be effective and might help shut down the ongoing epidemic in West Africa. More than 27,000 people were infected and 11,000 died, according to the World Health Organization.
Advertisement
“It’s not a drug, it’s not treatment, it’s a prevention tool”, said Baldet, who has over 20 years’ experience in research, surveillance and control of infectious diseases in Africa. “Having seen the devastating effects of Ebola on communities and even whole countries with my own eyes, I am very encouraged by today’s news”, said Norway’s Minister of Foreign Affairs, Børge Brende, who spearheaded a million global funding pledge to fight Ebola. But noted that the encouraging result obtained from the vaccine’s clinical trial does not mean it will head off future outbreaks.
“It is a game changer because there was nothing that could protect people against Ebola – no drug, vaccine or medicine”, says Dr. Marie-Paule Kieny, assistant director-general for health systems and innovation at the World Health Organization, who helped to lead the trial.
Of all the people assigned to get the vaccine right away after contact, none of them developed Ebola.
Though hopeful, the outcomes are “provisional” and the vaccine will not be accessible instantly as a community-wide Ebola guard, professionals admonished. However, it’s believed that this number will fall as it’s administered to more people. The Lancet said the Guinea trial would continue gathering evidence of VS-ZEBOV’s effectiveness and safety. As of July 26, there will be no more randomization, and none of the participants will be asked to wait three weeks.
Of the three nations, the virus was most active in Guinea (also where the epidemic began), so it provided scientists with an opportunity to test a new vaccine and, as it turned out, save the lives of those who received it.
Kimberley Steeds, Ebola vaccine trial team member, in the Ebola vaccine laboratory, Donka Hospital in Conakry, Guinea.
The vaccine, which was developed by Canadian government officials, is now licensed to Merck & Co., but is still pending approval from federal regulators.
“With such high efficacy, all affected countries should immediately expand vaccination of contacts of infected patients in order to break chains of transmission and vaccinate all front-line workers to protect them”, he said in a statement.
Advertisement
The trial was carried out using ring vaccination, which was used in the past to eradicate smallpox.