-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
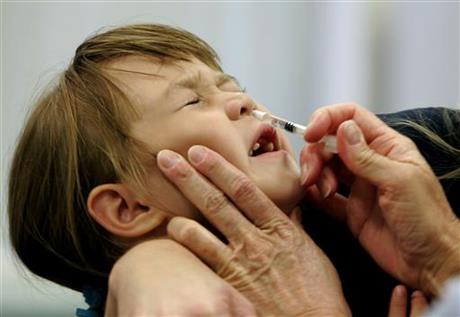
AstraZeneca takes $80 mln hit as USA spurns nasal flu vaccine
The updated guidance states the vaccine should not be used in any setting, based on CDC vaccine effectiveness data from the last three influenza seasons in the USA, which indicated FluMist Quadrivalent did not demonstrate statistically significant effectiveness in children 2-17 years of age.
Advertisement
The vaccine, FluMist, has not worked in the past three years, the panel was told by scientists from the Centers for Disease Control and Prevention.
Academy leaders say they support the interim recommendation by the CDC’s Advisory Committee on Immunization Practices (ACIP).
An interim recommendation from the Centers for Disease Control and Prevention (CDC) stated the vaccine did not show any statistically significantly effectiveness in children past year and should not be used in the 2016-2017 immunisation season.
FluMist uses live but weakened strains of the flu virus to stimulate immune systems, and is sprayed up the nose.
Experts were particularly anxious that FluMist hasn’t protected against H1N1, a type of flu that often causes more deaths and hospitalizations among children and young adults.
It’s not clear why the vaccine isn’t working. “We do understand this change will be hard for pediatric practices who were planning to give the intranasal spray to their patients, and to patients who prefer that route of administration”, said AAP CEO/Executive Director Karen Remley, M.D., M.B.A., M.P.H., FAAP.
Bresee said some suspect that it is has to do with the decision a few years ago to incorporate four strains of flu in FluMist instead of the traditional three.
An AstraZeneca spokesperson said the company is evaluating the committee’s recommendation.
Advertisement
The vaccine’s manufacturer, AstraZeneca, presented its own study that found the FluMist vaccine was somewhat effective, but still not did not work as well as flu shots.