-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
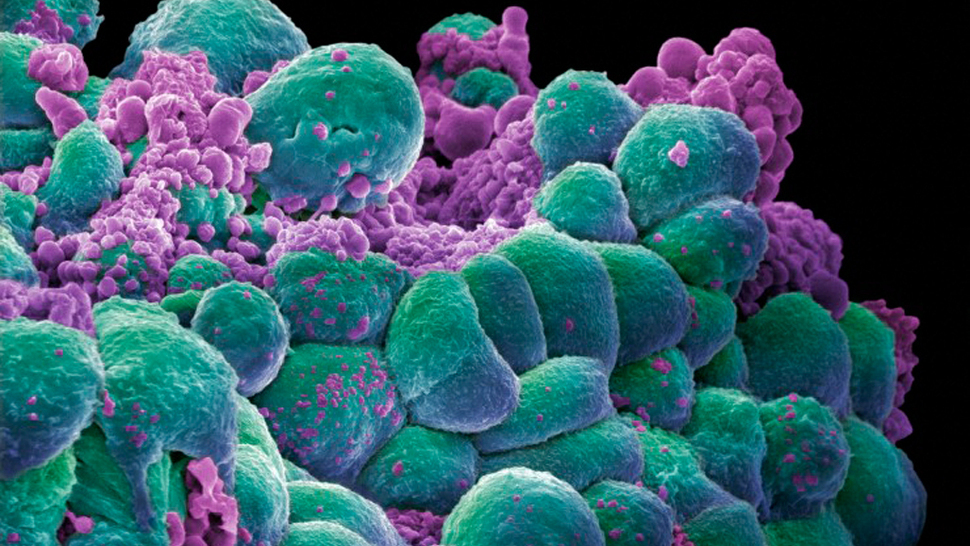
Juno (JUNO) Suffers Setback, Pivotal Study on Clinical Hold
The news of the trial’s suspension sent Juno’s stock down more than 30 percent.
Advertisement
Juno’s “Rocket” trial treats adult patients with relapsed acute lymphoblastic leukemia by injecting patients with engineered T-cells that attack cancer cells. The U.S. Food and Drug Administration (FDA) has ordered the 4-billion-dollar company to temporarily halt the promising study that was once expected to produce a breakthrough product.
He said Juno’s development of other products, including CAR-T therapy JCAR017, are not affected by the trial hold. All three patients died of a cerebral edema after receiving a drug called fludarabine as part of the pre-conditioning regimen.
Although no patient deaths in KTE-C19’s trials have been reportedly caused by KTE-C19, there was one patient death reported last August. Juno Therapeutics (JUNO) also receives 1 more Buy recommendations from analysts who believe that the stock will do well going forward.
Seattle-based Juno has called the addition of a second chemotherapy drug to its pre-treatment regimen responsible for the two recent deaths, instead of the new therapy of the company.
After two patient deaths, the government terminated an experimental treatment for leukemia.
The first experiments involved only one chemotherapy drug created to prepare the immune system for the experimental therapy.
In order to come up with the treatment in the market, Juno is competing with two other companies- Kite Pharma and Novartis. In a call Thursday, Juno said it’s now shooting for approval in 2018.
More than 12 million shares of Juno have already hands today, a substantial increase when compared to the stock’s average trading volume of approximately 1.1 million shares per day.
The treatment involves extracting immune cells, genetically altering them to kill cancerous elements, and injecting them back into the bloodstream.
Advertisement
In early stage clinical trials, CART therapies were found to eliminate all trace of lymphoma and leukemia in 40 percent to 90 percent of participants. Juno intends to submit the information next week. In fact, investigation into fludarabine was almost halted altogether for patients with leukemia, following the development of delayed central nervous system toxicity. Patients in the trial get a range of chemotherapy drugs while undergoing concurrent CART treatment, and in previous studies, Juno had some data that suggested the inclusion of fludarabine in this range of chemo drugs enhanced the efficacy of JCAR015. Junos product candidates, JCAR015, JCAR017 and JCAR014, utilize vehicle technology to target CD19, a protein expressed on the surface of various B cell leukemias and lymphomas.