-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
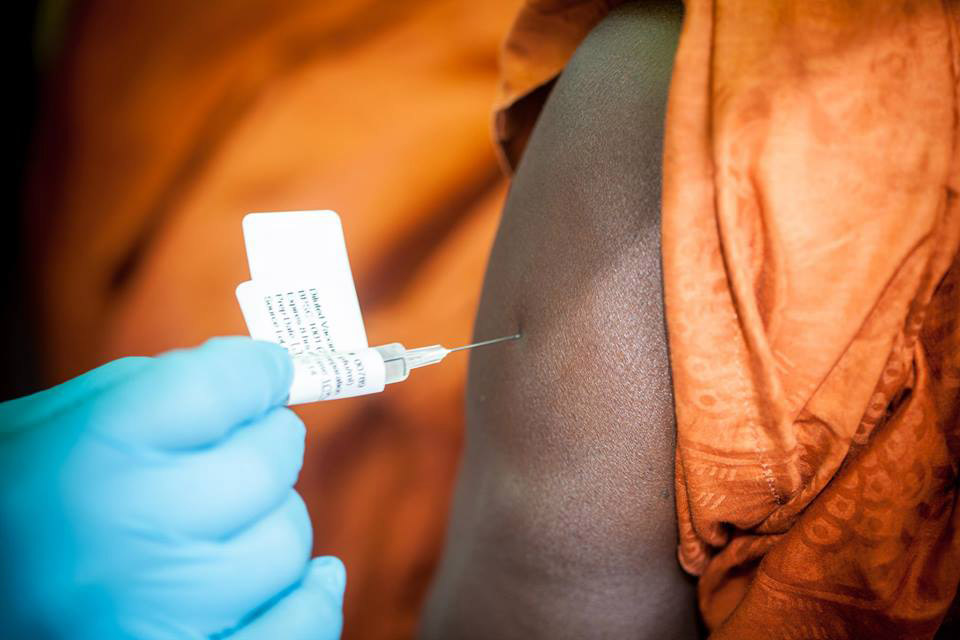
Vaccine against Ebola Virus proves 100% Effective
His excitement was shared by the scientist who headed the project to develop the vaccine, Dr. Heinz Feldmann, who struggled for years to try to find a way to prove that the vaccine would work as well in people as it does in non-human primates.
Advertisement
As of now, there are still other vaccines being tested, notably from Johnson&Johnson and GSK, although the trial period is becoming increasingly hard as the number of Ebola cases continue to dwindle.
Speaking to reporters Friday, UNMEER chief David Nabarro said he had seen signs that WHO had already absorbed some of the lessons of the Ebola disaster, recovering its co-ordination role in West Africa and deploying more 1,000 staffers to the field.
An Ebola test vaccine provided blanket protection in a field trial in Guinea, researchers said, possibly heralding “the beginning of the end” for the devastating West African outbreak that has killed thousands.
“If proven effective, this is going to be a game-changer”, said Dr. Margaret Chan, Director-General of the World Health Organization, which sponsored the study.
Because of the effective results, randomization for the trial was dropped on July 26, and everyone at risk was immediately given the vaccine.
And the cost of mass vaccination to cover all eventualities would be prohibitive.
“It looks to be about as safe as a flu vaccine”, said Ben Neuman, a virologist at the University of Reading who was not part of the trial. “We need to be ready to act wherever the virus is a threat”. The trial of the vaccine, called Ebola ca Suffit – “Ebola, that’s enough” in French – began on March 23.
Also today the United Nations (UN) announced the official end of its special mission for Ebola, the first ever geared toward a public health crisis.
The wrapped remains of a new born child suspected of contracting the Ebola virus, lays on a stretcher as health workers, dressed in Ebola protective gear, move the body for burial in Dubreka, Guinea, June 19, 2015. “With such high efficacy, all affected countries should immediately start and multiply ring vaccinations to break chains of transmission and vaccinate all frontline workers to protect them”.
Guinea’s national regulatory authority and ethics review committee says it plans to continue the trial based on these preliminary results, but it is still unclear what should happen next.
Some of the researchers involved with the study said that “they hadn’t expected such clear results”, explains Sheri Fink, a medical journalist who has been writing about Ebola for the New York Times.
The Global Alliance for Vaccines and Immunisation has approved substantial funding for the procurement and deployment of the vaccine.
“When there is a new outbreak this vaccine will be put to use to stop the outbreak as soon as possible to not have the awful disaster we have now”.
Ebola, which is a notoriously deadly virus, starts with high fever which could escalate into a massive hemorrhagic bleeding.
Seventy-two adults between the ages of 18 and 50 are participating in the trial, led by the pediatrics department at Oxford.
Advertisement
The vaccine was developed by Public Health Agency of Canada and then sold to the pharmaceutical company Merck to conclude the testing.