-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
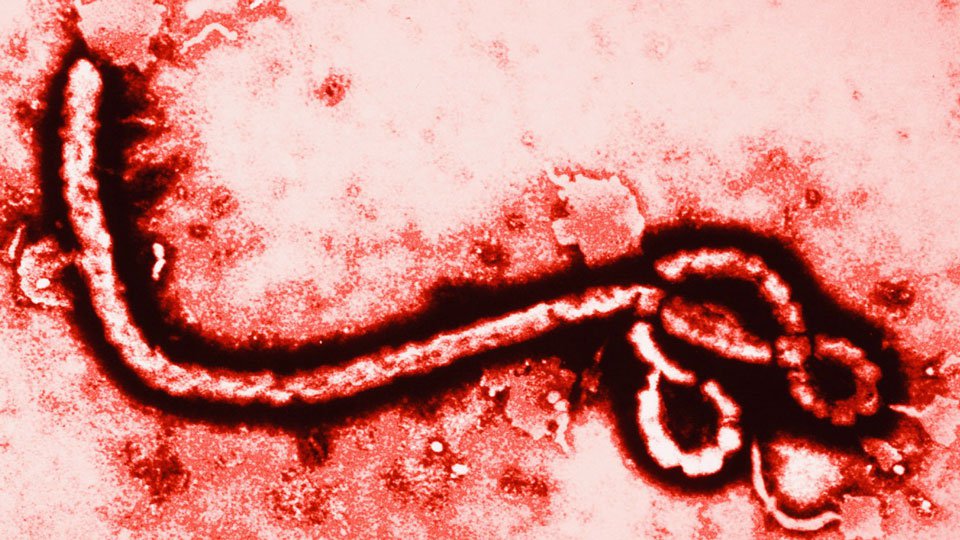
New Ebola Vaccine Is ‘Highly Effective’
The trial tested a vaccine called rVSV-ZEBOV, composed of a fragment of Ebola virus combined with another safer virus in order to boost the immune system to beat Ebola.
Advertisement
In addition, the researchers identified the people who developed Ebola during the study through the regular disease surveillance methods that are used in Guinea, but these methods might miss some cases, particularly if the cases were less severe, Goodman said.
VSV-ZEBOV was originally developed by Canada’s public health agency before being licensed to NewLink Genetics, which then signed a deal handing Merck the responsibility to research, develop, manufacture and distribute it.
Professor Andrew Easton, Professor of Virology, University of Warwick, who was himself treated for Ebola this year, said “More work on the data from this study is required and additional questions remain to be addressed”.
Jesse L. Goodman, professor of medicine at Georgetown University Medical Center in Washington, said the results published in The Lancet provided “exciting preliminary evidence” that the vaccine is likely to be effective but also cautioned that further analysis was needed.
When the biggest ever Ebola outbreak in the history of our planet began in December 2013, there was not a single proven vaccine or drug against the deadly virus.
The national regulatory authority and ethics review committee of Guinea have approved the continuation of the trials in the country. The results showed it to be so effective that as of 26 July, all trial participants are being given the vaccine immediately.
Because the virus is concentrated in “hot spots” across the region, it makes more sense to focus on vaccinating those close to infected patients and front-line workers than to embark on a mass vaccination campaign, he said.
The world is still cautious for the next Ebola outbreak but scientists are confident that with the current developments on the Ebola vaccine, the rate of mortality will not be as high as the previous outbreaks.
With case numbers waning, many experts saw the Guinea trial as the best hope of determining whether an effective Ebola vaccine was even a possibility.
The vaccine confirmed “100 % safety towards Ebola after roughly one week”, within the trial, researcher Sven Trelle from the College of Bern stated, in line with French information company AFP.
The trial vaccinated all 7000 people – some immediately and some after 21 days.
The results have been hailed as “extremely promising” by World Health Organisation chief Margaret Chan.
The vaccine, known as VSV-EBOV, was tested beginning in March on more than 4,000 people in Guinea who had come into close contact with the Ebola virus. The randomization has since stopped so that everyone can get vaccinated.
The hope now is that the legacy of this unprecedented outbreak will be a vaccine that means a tragedy of this scale can never be repeated.
Advertisement
Another WHO reform focus is on spearheading research and development work on vaccines, medications, and diagnostic tests, Chan said. The disease, which surged in the spring of 2014 primarily in Guinea, Liberia and Sierra Leone, has infected about 27,800 people and killed 11,300, according to the World Health Organization’s latest figures.