-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
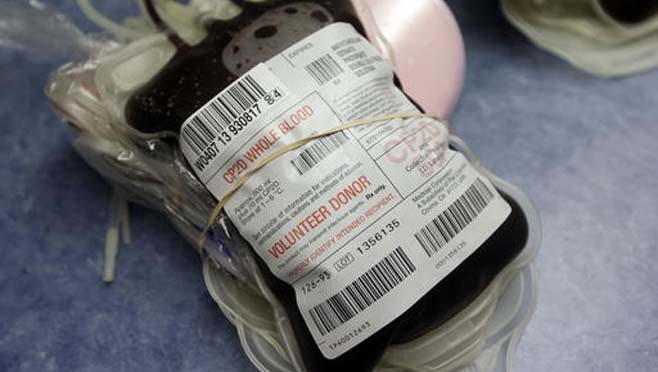
FDA Recommends Testing for Zika Virus in All US Blood Donations
All donated blood should undergo tests for the Zika virus, which can cause birth defects, USA regulators said Friday, amid a mounting outbreak of the mosquito-borne virus in the United States.
Advertisement
There have been almost 2,500 cases of Zika in the US linked to travel to outbreak areas.
“There is still much uncertainty regarding the nature and extent of Zika virus transmission”, Dr. Peter Marks said in a Food and Drug Administration release.
“At this time, the recommendation for testing the entire blood supply will help ensure that safe blood is available for all individuals who might need transfusion”, Marks said.
In February, the FDA recommended testing of donated blood and blood components only in areas where Zika was actively spreading, but agency officials now say that universal testing is needed to further protect those who get donated blood. A woman reported typical signs of a Zika infection 16 days after she had unprotected sex with her male partner, who had recently returned from the Dominican Republic, an area of active Zika transmission.
Dr. Luciana Borio, the FDA’s acting chief scientist, said that as the agency gets new scientific information about the disease, additional precautionary measures become necessary.
United States health officials Friday reported the first known case of a man who acquired Zika virus while traveling, showed no symptoms, and infected his female partner during unprotected sex.
Most of the cases within the continental USA involved individuals who contracted the virus while traveling in another country, while most of the Puerto Rico cases were locally acquired.
Zika virus infection during pregnancy has caused serious birth defects in a few cases in the USA and hundreds of cases in Central and South America where infants have been born with microcephaly, a condition where the brain and skull are malformed.
While the virus is mostly spread by the Aedes mosquito, sexual contact with an infected partner can also spread it.
The Community Blood Center applied in June for use of the investigational new drug (IND) authorized by the FDA as a blood screening test for Zika.
Around 2,200 Zika cases have been reported in the continental US and more than 13,000 in Puerto Rico, according to the Centers for Disease Control and Prevention.
With no FDA-licensed tests available for Zika, two experimental tests are being used to screen blood collected in the United States and Puerto Rico.
Anyone who has traveled to an area endemic with the Zika virus, especially in Central and South America, along with the Caribbean Islands, should seek medical attention if they develop any of the related symptoms, Hill said. The cost of adding Zika testing to the blood screening process is less than $10, according to officials at South Texas Blood and Tissue Center.
Blood banks already test donations for HIV, hepatitis, West Nile virus and other blood-borne viruses.
Advertisement
On Friday, DeLauro applauded to decision, calling it “a strong step forward in protecting our nation’s blood supply and the American people”.