-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
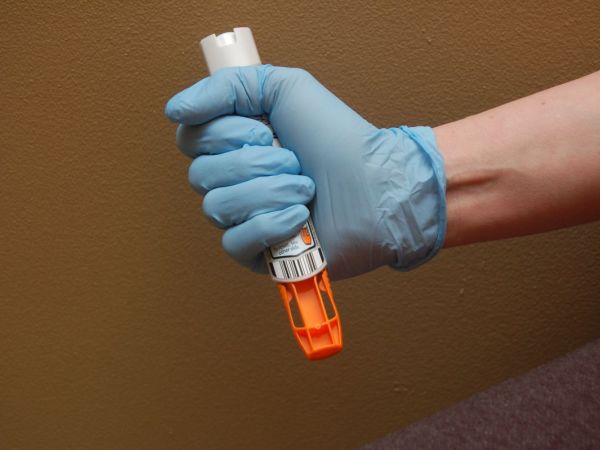
Mylan’s generic version could cost patients more, says pharmacist
The top two members of the House Committee on Oversight and Government Reform announced Monday they are officially launching an investigation into Mylan, the pharmaceutical company that drastically raised the price of the EpiPen, an emergency allergy shot.
Advertisement
Mylan said it expects to launch the generic alternative at a list price of $300, compared to the branded list price of $600. The drug price for a two-pack EpiPen has increased from $100 in 2009 to approximately $600 this year, according to medical literature and various pharmacies.
She notes, “Our decision to launch a generic alternative to EpiPen is an extraordinary commercial response, which required the cooperation of our partner”.
The announcement comes just four days after Mylan said it would offer patients a coupon and expanded financial assistance – a move referred to by some politicians and insurers as a superficial “PR fix”.
“Mylan knows its $600/set of EpiPens is unsustainable, but aims to continue ripping off some segment of the marketplace – both consumers who do not trust or know about the generic, and perhaps some insurers and payers constrained from buying a generic”.
“We would like the company to drop the price to $100 for two EpiPens”, said Public Citizen Spokeswoman Angela Bradbery, adding that was about the price charged in France.
“Mylan has a virtual monopoly over the epinephrine auto-injector market”, the lawmakers wrote.
EpiPen is a preloaded injection of epinephrine (adrenaline) used in case of a risky allergic reaction known as anaphylaxis that could cause death if untreated. Many other doctors do not even know the product’s name because of EpiPen’s long domination, he said.
“When its the manufacturer of the brand putting out their own generic product, it is going to be the exact same thing”, said pharmacist AJ Caraballo with The Hometown Pharmacy.
In addition, he said, “There will be heightened pressure on [the Food and Drug Administration] to bring competition to market”. One possible rival, Sanofi US, recalled its Auvi-Q epinephrine injector last fall because of dosage accuracy problems, while the launch of Teva Pharmaceutical Industries’ generic copycat was delayed.
CEO Heather Bresch has defended EpiPen’s high price, saying Mylan had spent hundreds of millions of dollars improving the product since acquiring it from Germany’s Merck.
U.S. health regulators rejected Adamis’s rival treatment to EpiPen in June.
Democratic presidential nominee Hillary Clinton and fellow legislators publicly blasted the latest move arguing that it is not enough.
Advertisement
Shares in the Dutch-incorporated Mylan, which is headquartered in England, were flat at $43.03 in early trade Monday after losing 12 percent last week amid the controversy.