-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
FSU Researchers Find Possible Solutions To Stopping Spread Of Zika
The compounds include emricasan, an investigational drug now being evaluated in a clinical trial to reduce liver injury and fibrosis, and niclosamide, a U. S. Food and Drug Administration-approved drug for use in humans to treat worm infections. “Independently of evaluating the potential benefits and risks for pregnant women, niclosamide could be used to reduce viral load in infected men and nonpregnnant women, which could reduce transmission and potentially prevent Guillain-Barre syndrome and other Zika-related complications in humans”, the study says. The team then evaluated the protective effect of these compounds in brain cells after Zika virus infection. Nationally, there have been 2,517 cases reported to the U.S. Centers for Disease Control and Prevention, including more than 40 attributed to local mosquito bites in Florida where the virus first hit the mainland United States.
Advertisement
SC confirmed 43 cases of Zika related to travel as of Friday, according to the S.C. Department of Health and Environmental Control.
Dr. Luciana Borio, the FDA’s acting chief scientist, said, “As new scientific and epidemiological information regarding Zika virus has become available, it’s clear that additional precautionary measures are necessary”. “We are issuing revised guidance for immediate implementation in order to help maintain the safety of the USA blood supply”.
Back in February, the FDA also asked that people who had traveled to places where the Zika virus is prevalent, or who have symptoms that suggest infection, should wait a month before donating blood. The drugs are either approved by the Food and Drug Administration or are being tested in final-stage clinical trials. This affects Florida and Puerto Rico. Friday’s directive lists 11 states – Alabama, Arizona, California, Georgia, Hawaii, Louisiana, Mississippi, New Mexico, New York, South Carolina and Texas – that will need to begin screening blood in the next month due to their location or because of the influx of travelers from Zika outbreak countries. The agency recommended that Maryland begin testing in 12 weeks.
Marks said Hologic Inc HOLX.O and Roche Molecular Systems ROG.S had been granted special approval for their tests to be used to screen the blood supply.
Researchers at the University of Texas Medical Branch at Galveston also looked at how existing drugs can treat Zika.
The FDA sent the recommendation stating that Georgia and SC would have to institute testing within 4 weeks unlike other states that have been given 12 weeks.
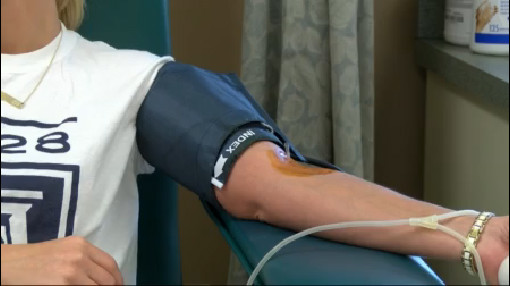
The revised guidance announced today recommends that all states and US territories screen individual units of donated Whole Blood and blood components with a blood screening test authorized for use by the FDA under an investigational new drug (IND) application, or licensed test when available. It is now being utilized in blood centres in the United States.
Oberlaender said, “As a leader in diagnostics, Roche is committed to providing testing solutions for the world’s most challenging healthcare emergencies”.
The scientists said the next step is to see if vertical transmission among mosquitoes occurs in nature. This drug is already FDA approved.
The Red Cross announced Friday it was conducting blood donor tests for Zika in five southeastern states and would be expanding testing to four more states in the next two weeks.
“What we’re hoping is that eventually the study either directly or through additional research will lead to some sort of affordable treatment for Zika virus infection that’s accessible for a lot of people”, Lee said.
Blood banks already test donations for HIV, hepatitis, West Nile and other blood-borne viruses.
Advertisement
“We are gathering data on the performance of the test while the test is in a sense being required by the FDA”, she said.