-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
Biogen’s aducanumab Fast Track’d for Alzheimer’s disease
Now, researchers hope that this new breakthrough drug called Aducanumab is able to hit the disease early enough to stop its progression.
Advertisement
People who eventually develop Alzheimer’s Disease have higher levels of certain immune system proteins in their blood, researchers find, indicating that a simple blood test could someday help predict our future risk.
Officials of the drug-develop Biogen were also careful about interpreting the findings of the study, which enrolled 165 patients in the initial stages of Alzheimer’s disease.
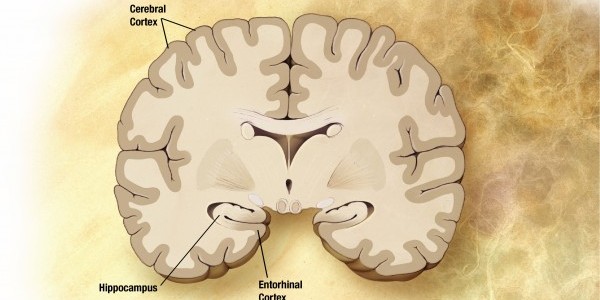
A NEW wonder-drug that halted Alzheimer’s in its tracks in early trials has been hailed as the “most promising” treatment yet for the disease. As the plaques collect, they begin interfering with the brain’s function, causing memory loss and other symptoms before terminating in death.
During the course of the disease, the brain shrinks dramatically, affecting almost all its functions.
Experts are taking care not to build up false hopes about the antibody drug, aducanumab, which clears away sticky protein fragments in the brain linked to Alzheimer’s.
While the trial was created to assess the safety of the treatment and not whether patients fared better on the drug, an “exploratory analysis” of the data revealed that the treatment appeared to slow the mental decline of patients who responded to the therapy.
Still, researchers warn, the trial was not created to show whether patients improved on the drug and the findings related to cognition are part of an “exploratory analysis” involving small numbers of patients.
The drug did have side-effects, however, including fluid build-up on the brain, and headaches.
Biogen has been working with aducanumab, an antibody that’s shown promise in Alzheimer’s treatment in the past. This has been suspected for some time, but has never been proven in humans.
Beta-amyloid plaque deposits in the brain are a classic sign of Alzheimer’s disease. Patients with a clinical diagnosis of early or mild Alzheimer’s were given monthly intravenous infusions of a placebo or aducanumab at four different doses for one year.
Scans showed that patients given the antibody showed a greater reduction in beta-amyloid. They found the clumps when analyzing the brains of people who died and suffered from the disease.
The success has now paved the way for more advanced human trials. The test, which was not designed for cerebral benefits, has improved the condition of patients who took the drug compared to those receiving a placebo.
Might we be one small step closer to treating Alzheimer’s disease?
And Dr James Pickett, Head of Research at Alzheimer’s Society, said: “These results are the most detailed and promising that we’ve seen for a drug that aims to modify the underlying causes of Alzheimer’s disease”.
An experimental drug dramatically reduced the toxic plaques found in the brains of patients with Alzheimer’s disease, a team reports in the journal Nature.
Advertisement
“As we keep going, we are getting closer and closer to finding what we need to know to make an intervention that will be meaningful”, says Dr. Marshall.