-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
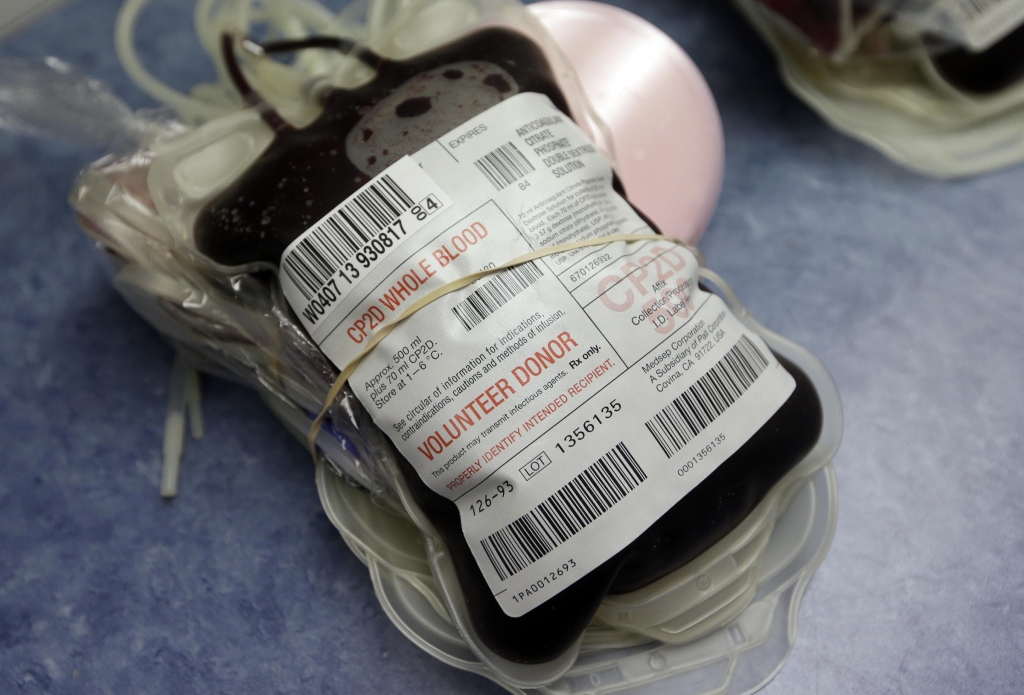
Donated blood across US to be screened for Zika Virus
All other states will have 12 weeks.
Advertisement
The Food and Drug Administration now recommends that all blood donations in the U.S.be screened for the Zika virus.
On the list: Alabama, Arizona, California, Georgia, Hawaii, Louisiana, Mississippi, New Mexico, New York, South Carolina and Texas. The most widely recognized source of the infection is through the bite of an infected mosquito.
Most of those cases were brought in by people who were infected while traveling overseas.
Right now, there are 2,517 confirmed cases of Zika in the US; 9,011 in USA territories. Yet two serum tests confirmed the presence of Zika antibodies in his blood.
Sabrina Cooper is a blood donor specialist and says Geisinger has already been testing for people with the Zika virus for several months now.
An executive for America’s Blood Centers, which has more than 600 locations in the USA and Canada, warned that the amount of work needed to comply with the FDA’s timeline is “titanic”. Adding Zika to other blood-borne viruses that collection banks already screen for, including HIV and the West Nile, costs less than $10 United States dollars.
This move comes after the FDA officially advised all blood banks to screen for the virus since its worldwide outbreak.
‘It has not yet been FDA licensed, so it is still in the investigational phase.
“The Zika virus is potentially a very bad threat, and I really do agree with testing patients that come in”, said Maldonado. Earlier this year, Puerto Rico had to shut down blood collections, costing the territory millions of dollars in imported blood from the mainland, Covin said.
“Overall, the number of persons with suspected [Guillain-Barre] and evidence of Zika virus or flavivirus infection was 2.5 times greater than the number of persons with suspected [Guillain-Barre] and no evidence of Zika virus infection”, the report says.
“There is still much uncertainty regarding the nature and extent of Zika virus transmission”, said Peter Marks, M.D., Ph.D., director of the FDA’s Center for Biologics Evaluation and Research.
“The transfusion of a pregnant woman with blood infected with the Zika virus could have awful consequences”, Peter Marks, director of the FDA’s Centre for Biologics Evaluation and Research, said. Marks said the concern is that they have been unknowingly infected and give blood.
Back in February, the FDA also asked that people who had traveled to places where the Zika virus is prevalent, or who have symptoms that suggest infection, should wait a month before donating blood.
Advertisement
The Zika virus has made headlines world wide. This news story is related to Print/145875-US-urges-all-donated-blood-undergo-tests-for-Zika/ – breaking news, latest news, pakistan ne.