-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
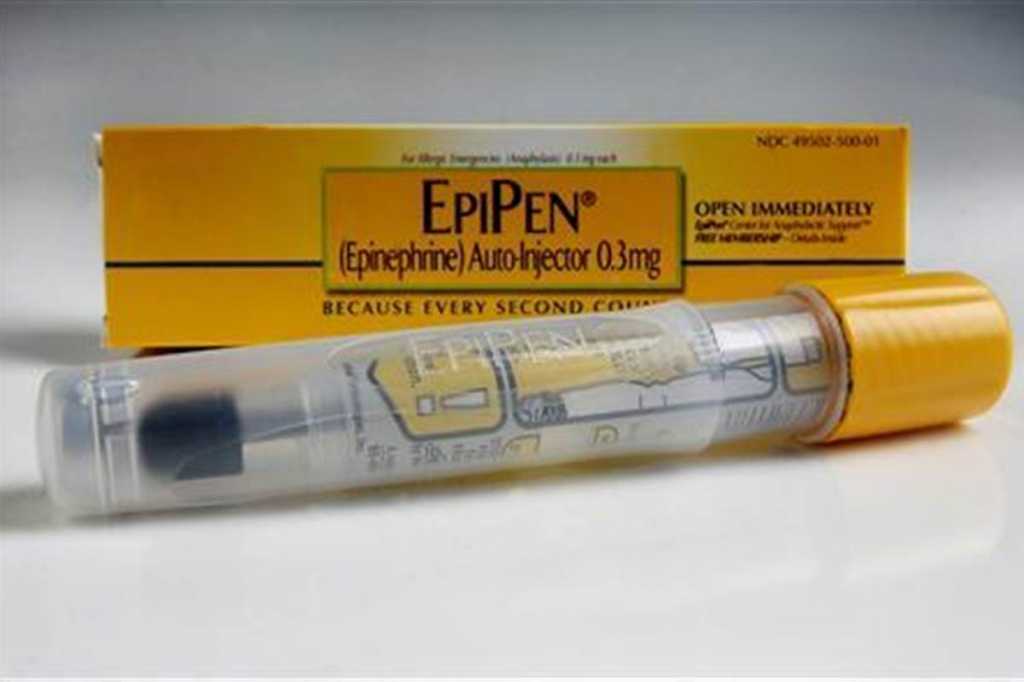
Local family concerned over rising cost of EpiPen
The only way to stop drug companies – such as Mylan in the case of the EpiPen – from jacking up the costs of live-saving treatments is through price controls, Dr. Ezekiel Emanuel told CNBC on Tuesday.
Advertisement
Global pharmaceutical company Mylan has announced it will launch a generic version of its EpiPen amid ongoing criticism over its recent 400 per cent price hike for its branded product in the United States.
To add to the injury, Mylan CEO Heather Bresch’s salary rose 671% to nearly $19 million annually from 2007 to 2015. In addition, Bresch was asked if Mylan prohibited EpiPen4Schools participants from buying any other brand of epinephrine auto-injector.
The authorised generic will be identical to the branded product, including device functionality and drug formulation.
In establishing a generic market, they’re also creating an early brand dominance for themselves, CNBC pointed out.
Another issue the senators are concerned about is whether Mylan will extend its patient assistance programs to include the authorized generic versions as well, or if not, how the company plans to ensure patient access to the generic. “I think prices would come down”, said Emanuel, one of the architects of Obamacare. On Thursday, Mylan reduced out-of-pocket costs of EpiPen for some patients, but kept the list price steady at roughly $600.
Mylan has responded to the public outcry over the price hikes by expanding programs to make EpiPens more affordable and promising a cheaper, generic version.
“This sounds like good news but the details are important to know”, said Republican Senator Chuck Grassley, who wrote a letter last week to Heather Bresch, Mylan’s chief executive, requesting further information on the cause of the price spike.
The senators said Mylan’s near monopoly on the epinephrine auto-injector market has allowed it to increase prices well beyond increases in manufacturing costs.
A company representative said Monday that the $300 cards would be available only for the branded version, but patients could use its assistance program for both the branded and generic versions of the medicine. None is likely to hit the US market until well into next year.
The introduction of Mylan’s generic also won’t automatically open the window to true competition from other generic companies, according to Michael Carrier, a professor at Rutgers Law School.
At least two companies are seeking approval to sell a rival brand or generic version of EpiPen in the United States, but none are likely to be available until later next year.
Advertisement
Updated from 7:57 a.m. with Monday share price.