-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
FDA tentatively approves first drug for muscular dystrophy
The approval comes almost five months after the Food and Drug Administration and a panel of outside advisers panned the drug, saying there was little evidence that it helped.
Advertisement
Duchenne muscular dystrophy drug – the first treatment for the degenerative disease – accelerated approval.
It’s the first drug to gain FDA approval for the condition. Under the accelerated approval pathway, the FDA is requiring Sarepta Therapeutics to conduct a clinical trial to confirm the drug’s clinical benefit. While no clinical benefit was found, approval was granted based on the disorder’s potential risks, its life-threatening nature and the lack of any other available therapy, the FDA said. About 13% of the 35,000 DMD patients in the U.S. and Europe have a mutation that might theoretically respond to a drug such as eteplirsen.
Duchenne’s muscular dystrophy is a rare disease, affecting about 1 of every 3,600 boys worldwide and usually causing death by age 25, according to the National Institutes of Health.
Pat Furlong, a patient advocate who lost two sons to the disease, called the announcement “an extraordinary win”. The drug acts on a protein called dystrophin, which plays a role in the growth of muscle fibers.
Until now there have been no FDA-approved drugs for DMD, and pressure has been increasing on the regulator to swiftly approve treatments.The FDA issued a scathing review of the drug in April, suggesting its perceived efficacy in a small number of patients could be due to chance because the company had not conducted a randomized, controlled clinical trial. The FDA is not required to follow the advice of its advisory panels, though it often does.
Advertisement
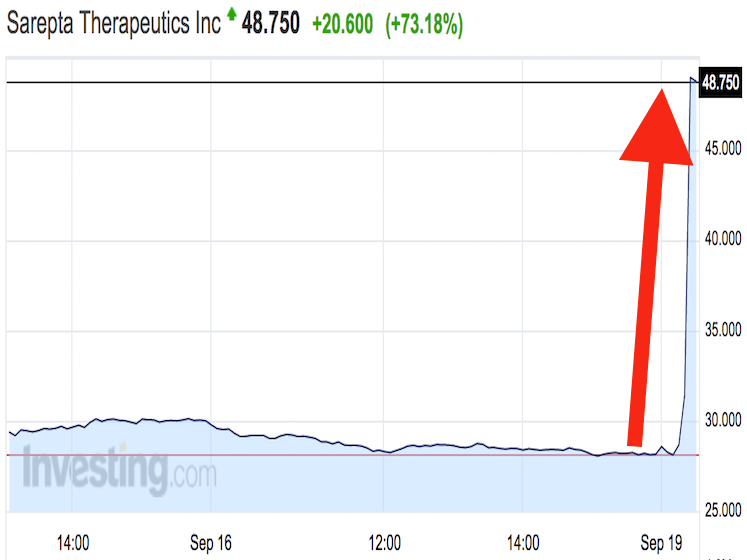
Shares in Sarepta skyrocketed by over 90% at one point Monday morning, as the FDA’s decision resolved a long-standing question of whether the company would be sent back to the drawing board. The gain gives Cambridge, Massachusetts-based Sarepta a market value of about $2.4 billion, and the positive FDA news could make it a much more appetizing – and expensive – takeover target.