-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
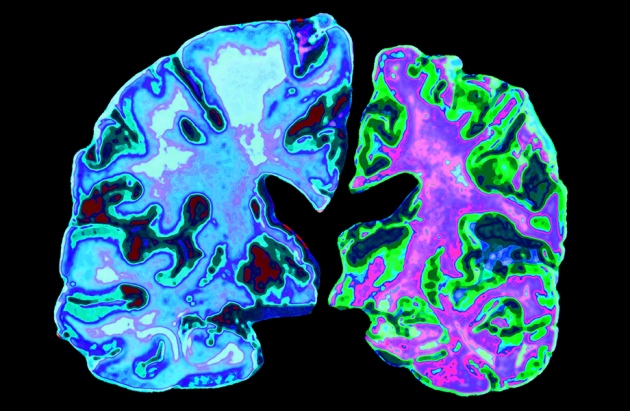
Another failure in search for treatment to slow Alzheimer’s
“This is probably not the end for amyloid approaches”, said Elizabeth Coulthard, a dementia specialist at the University of Bristol, who nonetheless acknowledged the late-stage Lilly trial result was “very disappointing”. For instance, beta-amyloid plaques also form in the brains of cognitively normal patients and the relationship between these plaques and declines in cognition aren’t well defined, according to a recent review.
Advertisement
“Crenezumab and gantenerumab are distinct from each other, as well as from other investigational medicines”.
Such an approach would require decades of treatment, an expensive and potentially risky endeavor if the therapy has even mild side effects that could emerge, or increase, over time, said Sam Gandy, associate director of the Mount Sinai Alzheimer’s Disease Research Center in NY.
The results raise questions about the integrity of amyloid as a treatment target in Alzheimer’s disease and may be a precursor for what is to come for similar agents and trials, including Biogen’s aducanumab and the A4 trial.
Analyst Geoffrey Porges of Leerink Research said that the finding is “a serious blow” for Biogen and other companies in the Alzheimer’s field, even though Biogen’s aducanumab is “a materially different antibody, in a different trial, at a different dose, and in different patients, than solanezumab”. There aren’t any drugs that effectively slow the disease’s progression, just ones that treat its symptoms. The Company will work with investigators to close the open-label extensions for EXPEDITION, EXPEDITION2, and EXPEDITION3.
Lilly said it would take a $150 million charge in the fourth quarter for the failed trial and on December 15 would update its 2016 financial outlook and provide a 2017 forecast.
A large study is testing the blood pressure drug nilvadipine.
No matter what Eli Lilly decides on its other solanezumab trials, the company isn’t giving up on Alzheimer’s.
An experimental drug to fight Alzheimer’s disease, called solanezumab, failed in a major clinical trial, the U.S. pharmaceutical giant Eli Lilly said on Wednesday (Nov 23), as experts called the results “disappointing”. “On behalf of over 5 million Americans with this disease and 15 million caregivers, we need an effective treatment”. Eli Lilly shares subsequently dropped 14 percent in premarket trading.
Widely anticipated results from a major Alzheimer’s trial have dashed hopes that a disease-modifying treatment may be around the corner. Those who took the drug did not have slower mental decline than those who took the placebo, BBC News reported.
One of the biggest hopes is a class of experimental drugs called BACE inhibitors, which work by blocking the beta secretase enzyme involved in producing beta amyloid. Eli Lilly says the results “directionally favored” solanezumab, but differences were so small that management has chose to shutter any future development of the drug. Drug maker Lilly designed solanezumab to target accumulation of amyloid protein, which forms sticky plaques in the brain of people with Alzheimer’s.
Advertisement
The dementia-causing disease has grown into the sixth-biggest cause of death in the USA, killing about 700,000 people annually, and is the only fatal condition among the top 10 in the US that can not be prevented, cured or slowed, according to the Alzheimer’s Association. In July, an experimental drug developed by TauRX Pharmaceuticals Ltd. failed to provide cognitive benefits.