-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
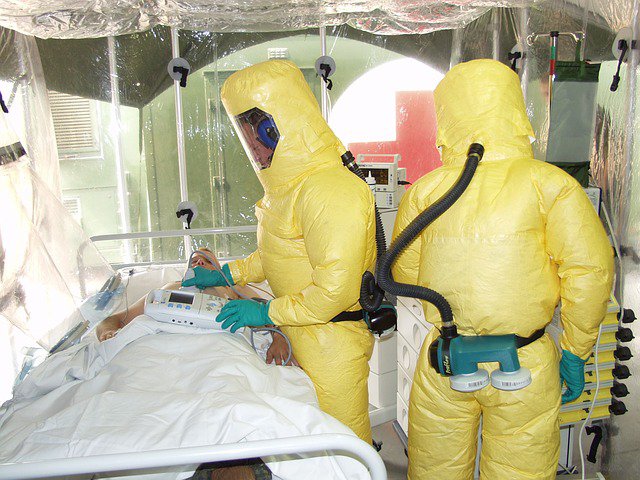
Guinea trial shows “historic” success for Ebola vaccine
Scientists on Thursday announced a milestone in the fight against Ebola, reporting that a major trial of an experimental vaccine shows that it may be “highly protective” against the virus, which has infected almost 30,000 people and killed 11,000 worldwide since 2013.
Advertisement
The vaccine has not yet been approved by any regulatory authority, but it is considered so effective that an emergency stockpile of 300,000 doses has already been created for use should an outbreak flare up again. All began with a sense of urgency but then petered out for lack of money.
Ebola was first discovered in 1976, and before the 2014 outbreak, it typically hit isolated African communities – those outbreaks were much more manageable for medical teams to parachute in and treat patients.
The trial was conducted on 11,841 citizens of Guinea this week.
World Health Organization assistant director-general Marie-Paule Kieny wrote in the medical journal “The Lancet” that an “effective vaccine” for Ebola, dubbed rVSV-ZEBOV should be 80 percent effective in a fully-fledged epidemic.
Over the next two years, more than 28,000 people fell ill, mainly in Guinea, Liberia and Sierra Leone.
Multiple health experts tested the vaccine in 2015 on nearly 6,000 people in Guinea. The company is expected to seek regulatory approval in the USA and Europe sometime next year.
But the virus reached cities in 2014, spreading like wildfire and catching the global health community off guard. The vaccine has flaws and limitations.
The post Final trial results confirm Ebola vaccine provides “high protection” – World Health Organization appeared first on BusinessDay: News you can trust. Joint pain and headaches have been reported as possible side effects. The situation emphasized the need for a vaccine that would alleviate the problem.
The trial was led by the WHO and the Guinean Health Ministry, Norway’s Institute of Public Health and other institutions. The protocol involves identifying and vaccinating people who have come into contact with someone who has an infectious disease.
Half got vaccine and half did not.
While it may have fallen from the news cycle, the Ebola outbreak in west Africa that led to the deaths of thousands of people between 2013 and 2015 has not been forgotten in the affected countries. Ebola virus has five known subtypes. In the group that did not receive the vaccine, 23 cases of Ebola were recorded.
There is no information on children, however, as the trial only included adult participants.
Advertisement
“We may have a vaccine which is registered in 2018”, Kieny told journalists at a press conference Thursday, noting that the standard approval process for a new drug takes a decade, if not more. Researchers say they used the same “ring vaccination” approach that helped wipe out smallpox, the Washington Post reports.