-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
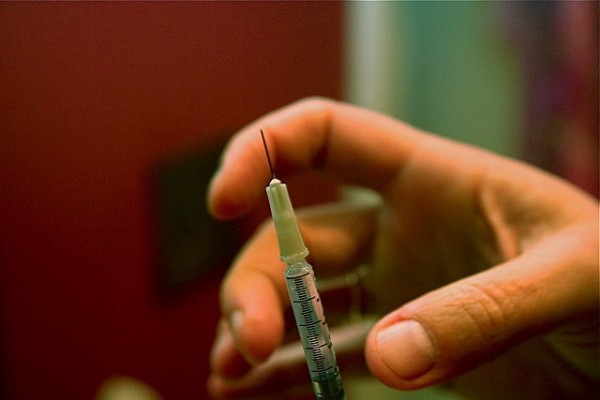
Alkermes’ treatment for Schizophrenia gets go-ahead
The FDA approved on Monday a new drug developed to treat schizophrenia called Aristada.
Advertisement
Alkermes plans to launch the new drug immediately, especially considering the schizophrenia treatment market is now pretty congested. The drug can be given once-monthly or every 6 weeks-the first atypical antipsychotic with this distinction, the company said in a statement.
Additional dosing options give Aristada a few ammo as it competes head-to-head with Otsuka and Lundbeck’s long-acting schizophrenia injection, Abilify Maintena. These features will help address the individual needs of patients and problems if any in the treatment of schizophrenia.
Alkermes also announced that they will keep focusing on central nervous system diseases, developing another schizophrenia drug, treatments for multiple sclerosis and major depressive disorder, according to Bloomberg.
David Henderson, M.D., of Massachusetts General Hospital said that despite numerous medicines, schizophrenia remains a serious and debilitating condition with “significant unmet medical need and suffering”.
The FDA’s certification was determined linked to confirmed take flight and performance rank, that were identified by way of files from random, double-blind, placebo-controlled, part a minimum of three reasearch which involve 623… Thomson Reuters Cortellis said Aristada’s peak annual sales could be $550 million by 2020.
Advertisement
FDA’s approval of the drug, however, comes with a boxed warning that states Aristada is not approved for patients diagnosed with dementia-related psychosis.