-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
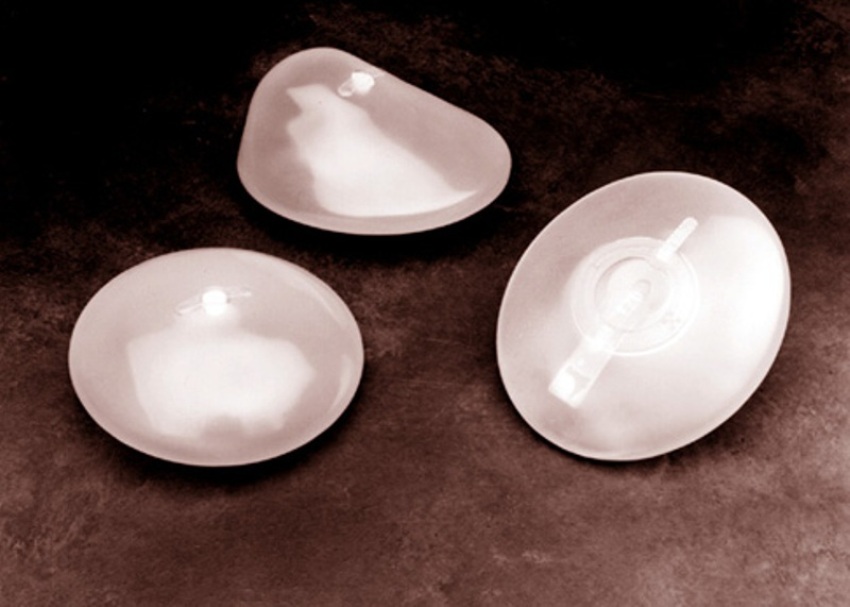
Brazil’s health regulator levies ban on production, sale and use of Silimed
According to the German declarations, the manufacturing surfaces that were subject to control were found to be contaminated with risky particles.
Advertisement
Brazil’s Health regulator Anvisa mentioned on Friday it had suspended the manufacturing, sale and use of merchandise made by Brazilian breast-implant maker Silimed after an inspection discovered the corporate failed manufacturing requirements.
In Brazil, the move was described as a precautionary measure to allow authorities to further establish the facts. The agency is still testing Silimed products and analyzing its manufacturing processes.
Breast implants in the South American country of Brazil are very common.
While an investigation is being conducted into what the particles are and how they ended up on Silimed’s products – which include silicone implants for breasts and other surgeries, implants for urology, and gastric bands and balloon bariatric surgery – the Brazilian health agency, ANISA, asked the company to shut down its production facility in Rio de Janeiro. No patients reported irregularities with the Silimed breast implants. It’s first in gross sales in Brazil, third on the planet and exports units to greater than seventy five nations worldwide.
In its announcement, ANVISA noted that no risks to patient health have been identified in connection with implanting Silimed products, and, accordingly, there is no need to adopt any procedure or action for those patients who have received them.
This news could be devastating to Silimed, which relies on sales in the United Kingdom to bolster its overall business. “All those questions are part of our current investigations”, he said.
Silimed – now working with Brazilian and European authorities to reverse the bans – asserted in a statement that it has always adhered to the highest standards of quality, and that the existence of sterile particles does not equate to a health risk.
The Silimed product suspension comes after medical authorities found in 2010 that one of the world’s leading breast implant makers, France’s Poly Implant Prothèse (PIP), was not using medical-grade silicone in its devices, leading them to have double the rupture rate of other implants. With thousands of implant users affected from Europe to South America, company head Jean-Claude Mas was given a four-year prison sentence in December 2013.
Advertisement
“There has been no indication at this time that these issues would pose a threat to patient safety”, said John Wilkinson, director of the MHRA, in a press release.