-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
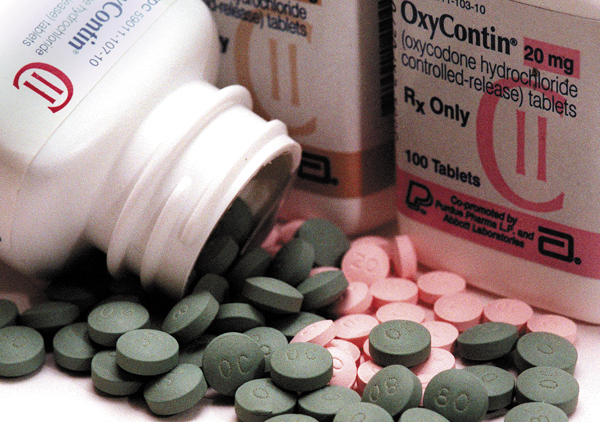
Children as Young as 11 Can Now Take OxyContin
OxyContin, the powerful and often-abused painkiller, has been officially approved by the Food and Drug Administration to use for children ages 11 to 16 who are suffering from severe, long-term pain. While many are finding it hard to believe that the FDA would allow such a drug for children, the administration’s Dr. Sharon Hertz, director of anesthesia and analgesia, gave a talk at the Center for Drug Evaluation and Research detailing just what OxyContin use for pediatric patients would look like.
Advertisement
The new approval also notes that doctors are only urged to prescribe OxyContin to pediatric patients who have been tolerant of other opioids in the past.
Doctors will now be directed to prescribe OxyContin only to children who can tolerate up to 20 milligrams of oxycodone, which is the primary ingredient in the painkiller.
Hertz said the agency requested the company do studies specifically with children because of the need for stronger drugs to treat pain in children – following procedures to correct extensive trauma, after major spinal or other surgery.
In order get approval of OxyContin for pediatric patients the FDA had to carefully identify how these drugs were being used and what information would be useful for prescribers.
According to Fox News, the FDA warning for pediatric patients is also applicable for adult patients.
Purdue’s chief medical officer, Gail Cawkwell, said in an emailed statement: “For the first time, physicians prescribing OxyContin for patients 11 years and older who have already been receiving opioids will be guided by FDA- approved dosing and safety information”. According to the agency, the manufacturing pharmaceutical brand of the drug was able to show valuable data that proved the medicine’s safety in pediatric patients if appropriately administered.
The duragesic patch, which releases fentanyl, is the other long-acting opioid option for pediatric.
The rates of OxyContin misuse and abuse remain high-in 2008, the number of new nonmedical users of OxyContin aged 12 or older was approximately half a million.1
.
Advertisement
The FDA’s Dr. Hertz said Purdue’s 2010 reformulation of OxyContin made it more resistant to being crushed or dissolved “to discourage abuse by nasal or intravenous routes”. “This way, their health care providers know that these pediatric patients can be treated safely with OxyContin”.