-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
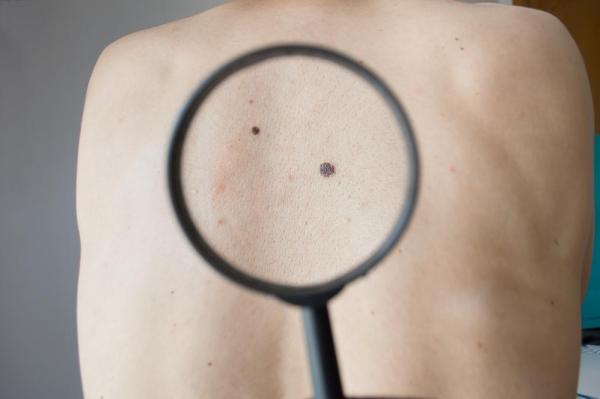
FDA approves drug for use in combination therapy for advanced melanoma
The drug combination used was found to extend the life expectancy of patients suffering from an advanced form of the disease without the existence of a side effect in the form of a secondary skin cancer, shown by patients who only took one of the drugs used in the new combination treatment.
Advertisement
Melanoma is aggressive and notoriously hard to treat. According to estimates from the National Cancer Institute, a total of 73,870 Americans in 2015 will be diagnosed with melanoma and 9,940 will die.
Commenting on the approval, the FDA’s Richard Pazdur said: “As we continue to advance our knowledge of tumour biology, we have learned that cancer cells have a remarkable ability to adapt and become resistant to targeted therapies”. It was tested on nearly 500 patients who suffered from an advanced stage of melanoma caused by a BRAF mutation and numerous patients participating in the trial showed an improved response to the combination of drugs than to the vemurafenib alone.
The drugs affect different parts of the same signaling pathway. As a result, cobimetinib prevents or slows cancer cell growth.
The FDA has approved Cotellic (cobimetinib) to be used in combination with Zelboraf (vemurafenib) for treatment of metastatic, non-resectable melanoma. Additionally, 65 percent of patients on the combination were still alive 17 months after starting treatment, while those only receiving vemurafenib had a 50 percent mortality rate.
Among patients given the combination, 70 percent saw complete or partial shrinkage of tumors, which only 50 percent of those taking vemurafenib and the placebo saw.
Severe adverse events may include cardiomyopathy, rhabdomyolysis, new skin tumors, retinal detachment, severe skin rash, liver damage, hemorrhage, and severe skin rash due to increased sensitivity to sunlight (photosensitivity).
The two drugs are Zelboraf and Cotellic.
COTELLIC is a selective inhibitor of MEK that was discovered by Exelixis Inc.
Cotellic was reviewed under the FDA’s priority review program that provides for an expedited six-month review of drugs that, at the time the application was submitted, have the potential to be a significant improvement in safety or effectiveness in the treatment of a serious condition. Dr. Ribas explained that the study found that when the experimental drug Cotellic is added, the combination works better and for much longer.
Advertisement
Both Cotellic and Zelboraf are marketed by Genentech, based in San Francisco. Conventional drugs typically get five years protection.