-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
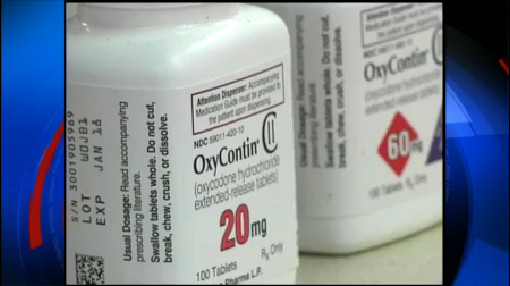
FDA approves OxyContin for kids
The long-acting drug already treats adults suffering from around-the-clock pain, and has been reformulated over the years to combat rising prescription drug abuse in the United States.
Advertisement
The new approval also notes that doctors are only urged to prescribe OxyContin to pediatric patients who have been tolerant of other opioids in the past.
Unlike adults, pediatric patients must already be responding to and tolerating a minimum opioid dose equal to at least 20 mg of oxycodone per day for 5 consecutive days before they can be prescribed an equivalent dose of OxyContin, Dr. Hertz said.
As to whether or not the drug is safe for use in children, Hertz says that when prescribing opioid drugs to pediatric patients, OxyContin should not be the go-to pain medication.
OxyContin, an extended-release version of the common opioid medication, oxycodone has gained an infamous reputation as a highly abused narcotic.
An FDA spokesman said the agency granted the approval Thursday, after the drug’s manufacturer, Purdue Pharma LP, submitted clinical-trial data on the drug’s safety and efficacy in children.
“We are always concerned about the safety of our children, particularly when they are ill and require medications and when they are in pain”, she said.
OxyContin, a synthetic drug similar to painkiller oxycodone, became popular in recent years because of its frequent misuse. The manufacturer also altered the OxyContin pill itself in 2010, making it more hard to crush and dissolve for snorting and injection.
Prior to the new OxyContin guidance, Duragesic-a form of fentanyl-was the only extended-release opioid specifically approved for pediatric use by the FDA. This is after a study that the FDA told Purdue Pharma, the drugs creator, to conduct regarding pediatric use.
OxyContin accounted for about 2 percent of the 215 million prescriptions written for opioids last year, according to IMS Health. The pills and tablets are formulated to slowly release their drug contents over 12 or more hours.
Advertisement
NIDA did not comment directly on the new OxyContin guidance but strongly reiterated the need to monitor any patient who receives prescription opioids. “When appropriate, the doctor can then convert their patient over to an OxyContin dose that is tailored to their individual needs”.