-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
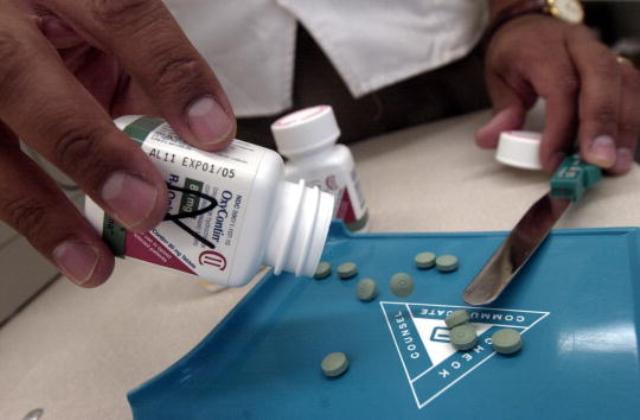
FDA approves use of OxyContin in children ages 11-16 years
The Food and Drug Administration has officially approved the use of OxyContin in children ages 11 to 17, which is a huge change since originally, the drug was only able to be administered to adults.
Advertisement
Doctors will now be directed to prescribe OxyContin only to children who can tolerate up to 20 milligrams of oxycodone, which is the primary ingredient in the painkiller. It is hard to find out real impact of opioids because a large percentage of death certificates do not disclose which drug is accountable for the drug overdose, Botticelli added. The most frequent adverse events observed in pediatric patients were vomiting, nausea, headache, pyrexia, and constipation. According to the federal agency of the United States Department of Health and Human Services, only limited use of the drug has been approved. Reports of all postmarket adverse events occurring in children aged ≤17 and younger related to respiratory depression, accidental injury, overdose, misuse, accidental exposure, and all medication errors, regardless of outcome, are also mandated.
OxyContin is manufactured by pharmaceutical company Purdue Pharma of Stamford in Connecticut and is an extended-release type of the opioid medication called oxycodone.
The duragesic patch, which releases fentanyl, is the other long-acting opioid option for pediatric pain management.
The previously abused painkiller OxyContin is a version of oxycodone drug, which is extremely powerful. The studies Purdue conducted led the FDA to approve OxyContin to treat pain in children 11 to 16 years old. The final study is due April 2019.
“To manage pain in pediatric patients, physicians often have to rely on their own experience to interpret and translate adult data into dosing information for pediatric patients”, said Dr. Sharon Hertz, director of the Division of Anesthesia, Analgesia, and Addiction Products at the FDA, in a press release. Medical experts continue to disagree over the appropriate role of the drugs, with some arguing that they should be reserved for the most severe cases, such as cancer pain or end-of-life care.
Advertisement
OxyContin was the first in a class of long-acting opioids designed to deliver powerful, around-the-clock pain relief.