-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
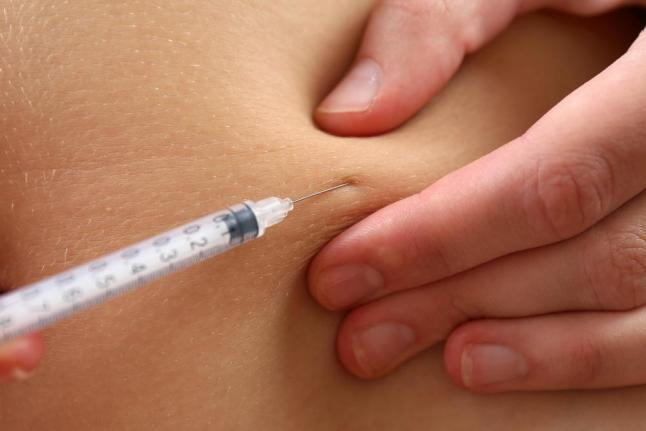
FDA OKs Breakthrough Cholesterol Drug
Praluent is approved for use in addition to diet and maximally tolerated statin therapy in adult patients with heterozygous familial hypercholesterolemia (HeFH) or patients with clinical atherosclerotic cardiovascular disease such as heart attacks or strokes, who require additional lowering of LDL cholesterol.
Advertisement
But the prospect of introducing highly-expensive, injectable drugs for one of the most common medical conditions is drawing concerns from health insurers, doctors and employers.
More important, the drugs seemed to cut the risk of heart attack or death from heart disease, as indicated by the researchers. Sanofi, which makes the drug, said in a press release it plans to charge about $40 per day for it – ten times the daily cost of generic stains – which works out to more than $14,000 per year.
“We don’t want the noise about these drugs to be price”, says Leonard Schleifer, the chief executive and founder of Regeneron. He noted that estimated costs for a patient who has suffered a heart attack or similar cardiovascular problem range from $50,000 to $119,000 over 1 year. “In the meantime, we continue to recommend physicians limit prescribing to the very high risk, hard-to-treat group approved by the FDA”.
Experts did urge some caution, however: The trials so far have been short-term, and it’s not clear whether the new cholesterol drugs really do extend people’s lives, reported by Dr. Seth Martin, a cardiologist at Johns Hopkins University in Baltimore. “I think the wisest thing is to be cautious about their use and reserve them for people who have no other choice”.
In terms of the large primary prevention population without FH, “I can understand why the FDA may have shied away from statin intolerance”, said Howard S. Weintraub, MD, clinical director of the NYU Center for the Prevention of Cardiovascular Disease in New York City.
The FDA has a target action date of August 27, 2015, under the Prescription Drug User Fee Act for review of evolocumab. Pfizer’s entry into the field is expected to launch in 2018 or later.
The FDA is also considering another cholesterol-lowering option. It’s the first in a new class of drugs that remove LDL cholesterol from the blood. Participants taking Praluent had an average reduction in LDL cholesterol ranging from 36 to 59 percent, compared to placebo.
Alirocumab is only prescribed for people who have reached a maximum dose on statins but still need lower cholesterol levels. But definitive studies are still ongoing.
The FDA, an agency within the U.S. Department of Health and Human Services, protects the public health by assuring the safety, effectiveness, and security of human and veterinary drugs, vaccines and other biological products for human use, and medical devices.
For one, the drugs have to be self-injected, which might put some people off. On the other hand, Martin said, the injections are done only once a month or every couple of weeks.
Now, each company is expected to mount a major promotional push to get its new therapy off the ground and onto the market, but the PCSK9 class is likely to face pushback on price from payers.
Advertisement
Pharmacy benefit manager, CVS Caremark, has warned that if 10 million U.S. patients ultimately take PCSK9 drugs it could result in well over $100 billion in new drug spending.