-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
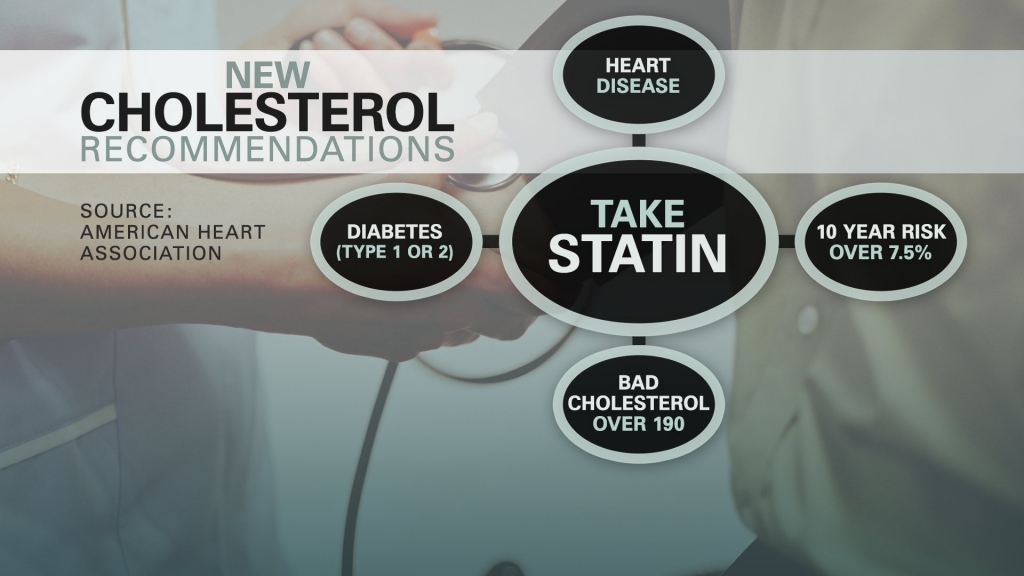
FDA OKs breakthrough cholesterol drug for high-risk patients
The U.S. Food and Drug Administration said Friday it has approved a breakthrough drug that lowers bad cholesterol more than older medicines that have been prescribed for decades.
Advertisement
Alirocumab (Praluent, Sanofi/Regeneron) is an antibody targeting PCSK9, a protein that reduces the number of receptors on the liver that remove LDL from the blood, as reported by an FDA press release.
Most people who have muscle problems from statins do not have those elevated muscle enzymes, says Nissen, the Cleveland Clinic cardiologist, who is conducting a clinical trial Amgen and Pfizer, which is also developing a similar cholesterol drug.
Reported by the label, Praluent is approved for patients with inherited very high cholesterol, a condition known as heterozygous familial hypercholesterolemia, or with established clinical cardiovascular and who can’t achieve desired LDL levels on statins alone.
The drug’s $40-a-day price is even higher than many analyst predictions, which centered on $10,000 per year. They must be self-injected by patients, with Praluent dosed every two weeks.
The FDA has a target action date of August 27, 2015, under the Prescription Drug User Fee Act for review of evolocumab.
“While the FDA’s focused guidance recognizes the safety and effectiveness of this treatment for certain patients, the exorbitant price raises concerns as to whether consumers and the health system can sustain the long-term costs”, the group’s interim chief executive, Dan Durham, said in a statement. “When you consider that cancer will affect one in three individuals over their lifetime, and [with] recent trends in insurance coverage [that] put a heavy financial burden on patients with out-of-pocket expenses, you quickly see that the situation is not sustainable”.
But analyst predictions for the drugs are more modest, at least initially.
On July 21, European regulators approved Repatha with a broad label covering patients with all familial hypercholesteremia, including the rare, homozygous form, and those with non-hereditary high cholesterol. Amgen’s shares fell 3.4 percent to $158.59.
“Despite statins and other lipid-lowering therapies, many patients are unable to reach their LDL cholesterol goals, and may benefit from new therapeutic options such as Praluent”, said Dr.Elias Zerhouni, president of research and development for Sanofi and a former director of the National Institutes of Health. Mutations in the gene that makes it can result in patients having lower cholesterol and a dramatically lower lifetime risk of heart attacks. A survey of physicians published this morning by Geoffrey Porges, an biotechnology analyst at investment bank Sanford C. Bernstein, found that they would use the drug in 30% to 40% of patients who had already had heart attacks.
Advertisement
Access Investor Kit for Gilead Sciences, Inc. Gilead was forced to discount its price after a rival drug from AbbVie Inc emerged late past year. The injectable drug, from Regenron and Sanofi, is the first in a new class of drugs called PCSK9 inhibitors.