-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
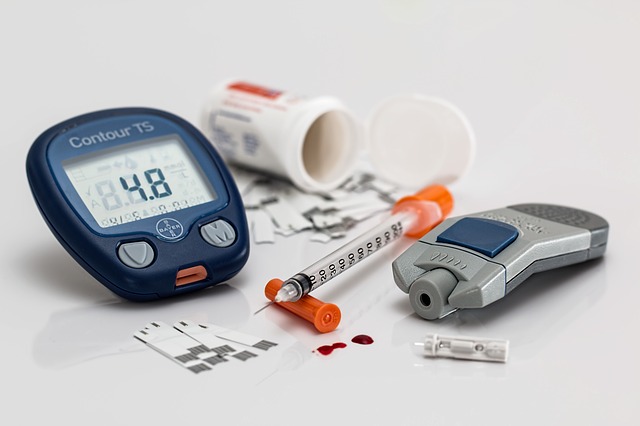
FDA OKs New Injectable Type 2 Diabetes Medication
While good news for many diabetes sufferers, the treatment is distinctly not for those suffering type 1 diabetes or those with diabetic ketoacidosis. Additionally, severe hypersensitivity reactions like anaphylaxis have been reported in clinical trials.
Advertisement
“The approval of Adlyxin reaffirms our continued commitment to addressing the challenges faced by people living with diabetes…” said Peter Guenter, Executive Vice President and Head of Sanofi’s Global Diabetes & Cardiovascular Business Unit, today in a statement.
Furthermore, data from a cardiovascular (CV) outcomes trial showed that lixisenatide did not increase the risk for adverse CV events in 6000 patients with type 2 diabetes who were at risk for atherosclerotic CVD. All studies of the GetGoal programme successfully met the primary efficacy endpoint of HbA1c reduction.
Adlyxin will be available in a disposable pre-filled pen in a single dose of 20 micrograms. Other adverse effects included nausea, vomiting, headache, and diarrhea. Patients will also receive a pre-filled pen containing a single 10 mcg dose that they should initiate once daily for 14 days before transitioning to 20 mcg. The therapy has been sold as Lyxumia in more than 60 countries, including most of Europe, as well as Mexico and India, which have high rates of diabetes. Commercial launches include most European Union countries, Japan, Brazil, Mexico and India.
Adlyxin is a once-daily glucagon-like peptide-1 (GLP-1) receptor agonist that works by increasing glucose-dependent release, decreasing glucagon secretion, and slowing gastric emptying.
The drug blocks the effect of glucagon-like peptide (GLP-1) on pancreatic cells that, when functioning normally, regulate insulin secretion.
Advertisement
Type 2 diabetes affects more than 29 million people in the United States, accounting for about 90 percent of diagnosed cases of diabetes, the agency said.