-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
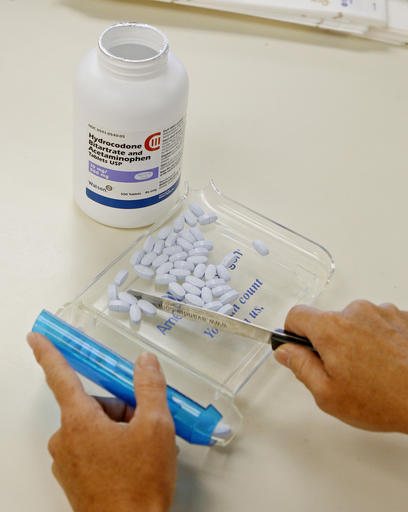
FDA Prescribes Safety Warning Changes for Opioid Painkillers
Those medications are among the most commonly prescribed drugs in the United States – accounting for 90 per cent of all opioid painkillers.
Advertisement
The so-called “Black-box” warnings are the toughest the agency can require.
Among the changes, the FDA requires a new boxed warning about the serious risks of misuse, abuse, addiction, overdose and death.
Short-acting opioid painkillers will carry strong new warnings under U.S. Food and Drug Administration requirements announced on Tuesday that will bring information about addiction and abuse in line with that on long-acting pills.
“This epidemic touches all corners of our nation and is devastating individual lives, communities and our nation”, said FDA Commissioner Robert Califf during a briefing for reporters.
Under the new guidelines, doctors are advised to avoid prescribing painkillers as a first-choice for common ailments, such as back pain and arthritis.
Markey pledged to continue to work with lawmakers to pass legislation that ensures all prescribers of opioid pain medication are educated in safe prescribing practices and the identification of possible substance use disorders. Certain opioids, such as methadone and buprenorphine, are also used as a form of treatment for opioid addiction, and in combination with behavioral therapy and counseling, are known as medication-assisted treatment, or MAT.
Prescription opioids often combine oxycodone with lower-grade medications. Almost 2 million Americans aged 12 or older either abused or were dependent on prescription opioids in 2014, according to the Centers for Disease Control and Prevention.
Required safety labeling language can be found here.
The FDA and Califf in particular have come under fire from some senators who have been highly critical of Califf’s role in what they said is an underwhelming agency response to the prescription opioid epidemic.
Throckmorton said the agency’s 2013 labeling change focused on long-acting drugs like OxyContin because they represented a “disproportionate risk” to patients, since they contain larger opioid levels.
Dr Doug Throckmorton, a deputy director in the FDA’s drug center, said: ‘This new indication, once finalized, will remind prescribers that immediate-release opioids are also powerful drugs with important safety concerns’.
The latest requirement brings the labels for all opioids into alignment.
Government officials have tried a variety of approaches to tackling painkiller abuse in recent years.
We don’t fill them up early.
And, Florida and NY have cracked down on “pill mills” by using databases to monitor what doctors are prescribing.
The Obama administration has asked Congress to provide $1.1 billion to combat opioid addiction.
The boxed warning also requires a precaution that describes how chronic maternal use of opioids during a pregnancy can result in neonatal opioid withdrawal syndrome (NOWS), a potentially fatal condition a newborn may be exposed to if opioid drugs are consumed by the mother over a prolonged period while the infant is in utero.
Advertisement
The label will further include information about drug interactions with antidepressants and other medications. Here’s a National Pain Report story on the CDC guideline.