-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
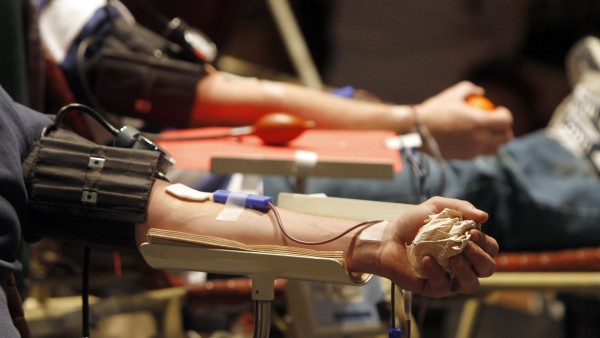
FDA Recommends Testing All Blood Donations for Zika
Previously, the FDA had limited the requirement to Puerto Rico and two Florida counties. “At this time, the recommendation for testing the entire blood supply will help ensure that safe blood is available for all individuals who might need transfusion”. “Eighteen countries and territories have reported an increased incidence of Guillain-Barre syndrome (GBS) and/or laboratory confirmation of a Zika virus infection among GBS cases”, the group said.
Advertisement
Prijatel said Medic has been “proactively planning to implement changes to allow for Zika testing”, which the FDA said Tennessee and Kentucky blood centers must do within the next 12 weeks.
Currently, Zika is being spread by mosquitoes in South Florida, Puerto Rico and the U.S. Virgin Islands, as well as most countries in the Caribbean and Central and South America.
CBC will continue the screening methods in place to defer potential donors who have traveled to Zika endemic areas in the prior four weeks.
“The FDA released today it’s recommendation to begin testing on all donated blood for the Zika Virus”, says Tina Hooper, LifeShare Blood Centers Executive Director Marketing/Communications.
Obama’s latest appeal, in his weekly radio address, came the day after the USA authorities expressed deepening worry about the spread of the mosquito-borne virus, urging that all donated blood be tested for Zika.
“We ask each blood donor who comes in to donate whether it be here or at a mobile blood drive, if they’ve travelled to Florida in the last 30 days”, said Media Relations Representative Chris Pilgrim.
Zika is mainly spread by mosquitoes, but a person can also contract the virus through unprotected sex with an infected partner.
Without federal funds, it’s usually hard for local health departments to conduct active surveillance for Zika virus in the blood or urine of individuals with fever or rash, Dr. Peter J. Hotez added.
Bowman says the process from the FDA to blood centers can be arduous; and the extra steps in testing can come with more costs, and more work.
In the other 21 US cases of sexual transmission, the virus was spread by someone who at some point had symptoms.
Advertisement
“We are issuing revised guidance for immediate implementation in order to help maintain the safety of the USA blood supply”, noted Borio. Lloyd Doggett, D-Texas, Patrick Murphy, D-Fla., and a half-dozen other House members, in a letter to the FDA earlier this month. Two women in California gave birth to two babies with microcephaly, a condition in which a child’s head is significantly smaller than expected.