-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
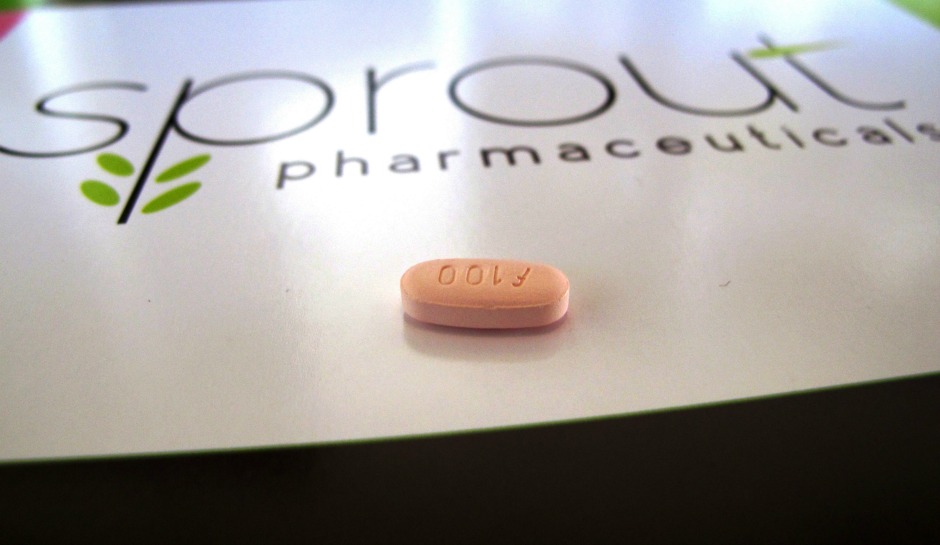
Female Libido Drug, Addyi, Shows Modest Results
Women may experience small benefits with a drug approved a year ago in the U.S.to treat low sexual desire in women, but at a high risk of experiencing unpleasant side effects, according to a new analysis. After analyzing eight clinical trials involving more than 5,900 women, Dutch researchers at Erasmus University Medical Center in Rotterdam found that one in three women reported negative side effects like nausea, fatigue, dizziness and insomnia.
Advertisement
Women taking flibanserin had about one-half of one more satisfying sexual encounter per month, compared to women taking a placebo or “dummy” pill, the research showed. Researchers actually suggested that experts hold off recommending the drug until future studies are conducted to prove its worth, writing: “The findings of this review suggest that the benefits of flibanserin treatment are marginal, particularly when taking into account the concurrent occurrence of adverse events”.
“The FDA approved a marginally effective drug for a non-life-threatening condition in the face of substantial, and unnecessary, uncertainty about its dangers”, Dartmouth Institute for Health Policy and Clinical Practice’s Dr. Steven Woloshin and Dr. Lisa M. Schwartz commented via MNT. All told, the new analysis reflects the drug’s safety and effectiveness as tested in some 5,914 women.
The drug was also concerning to researchers because women taking it must avoid alcohol, or risk developing dangerously low blood pressure.
The drug, formally called Flibanserin, is also known as Addyi.
Post-marketing studies required by the FDA at the time it approved flibanserin won’t be done for between a year and 2 1/2 years. The drug had been rejected twice by the FDA before receiving approval, and still faced criticism when it was cleared to market last summer.
An editorial accompanying the article, published in JAMA Internal Medicine, questioned the FDA’s approval of the drug.
Despite criticism of the female libido pills, some doctors and patients say the drug has helped. However, he noted that reviewing the existing data does not carry the same weight as the clinical trials. Currently, there is no other FDA-approved alternative for women with low sex drive. On the contrary, one new study suggested that one in nine who take the drug might have a genetic variation that could cause fainting after just one dose. “We all need a drug approval process that delivers good decisions based on adequate evidence”.
Advertisement
The study authors wrote in JAMA Internal Medicine: “With almost 90 percent of American physicians indicating that they would prescribe an approved hypoactive sexual desire disorder pharmacological product over available nonpharmacological treatments, the need for sound evidence on the efficacy and safety profile of flibanserin is evident”.