-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
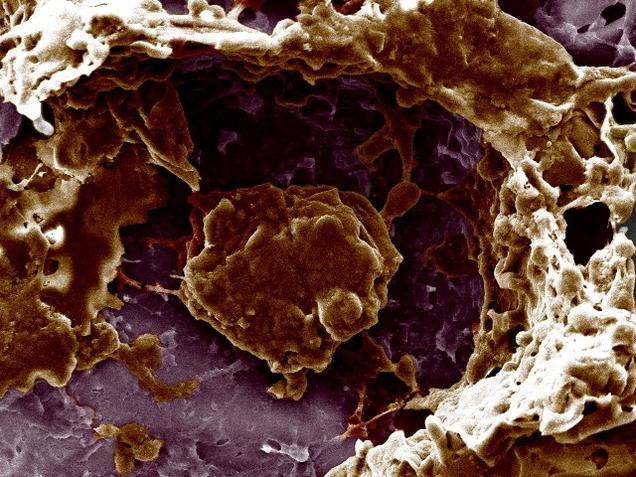
Gene-Editing Tool Approved For Use In Humans
Although the green light from the US Food and Drugs Agency (FDA) must be received before the trial can start, this week’s approval by the advisory committee to the National Institutes of Health, brings the technology a step closer to clinical use – and sooner than widely anticipated.
Advertisement
The trial will involve up to 15 patients. The team will now have to convince regulators in the USA and their own institutions to allow the trial which is expected to start by the end of the year.
The small study, which has been funded by ex-Facebook president Sean Parker, will augment T cells – potentially a way to treat cancer – but will be used to see whether Crispr is safe in humans, rather than being used as an actual treatment. T-cells are part of the body’s immune system, assisting in cell-mediated immunity.
CRISPR, the acronym of Clustered regularly interspaced short palindromic repeats, are segments of prokaryotic DNA containing short repetitions of base sequences. “Also it should not trigger off unexpected things, such as autoimmune disorders”, said Dr C.S. Mani, Surgical Oncologist with the Madras Cancer Care Foundation and Cancer Research and Relief Trust, Chennai.
The experiment targets difficult-to-treat cases of multiple myeloma, sarcoma, and melanoma.
The technique will remove T-cells, inject a manufactured gene to target cancer cells, and remove separate genes that can interfere with the process.
Advertisement
However, its preclinical activity had caused much hype given its gene-editing capabilities, with its core tech centred on a simple method for reengineering DNA – which may be expected to have profound consequences in treating a number of diseases. Second, knock off a naturally occurring gene in the T-cell which can hinder this. The point of the former is clear – to release a potential brake on immune system activity – but the latter less so. While the treatment shows promise, “our efforts have been hampered because the infused T cells become exhausted and die or cease to function”, the scientists said in explaining their proposal. According to STAT, the trial would be conducted at MD Anderson Cancer Center in Texas, with nine patients enrolled; the University of California, San Francisco, with three patients; and an additional three patients enrolled at the University of Pennsylvania.