-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
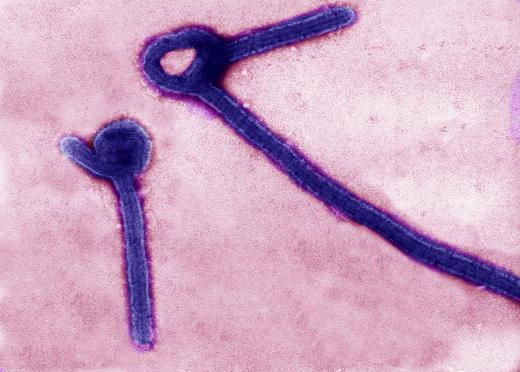
Global Fund For Vaccines Can Prevent Outbreak Of Deadly Diseases
In March, clinical trials started in Guinea, Africa.
Advertisement
The virus that killed more than 11,200 people in West Africa since it began in December of 2013, being the worst outbreak recorded, may now be able possibly extinguished.
How did the vaccine come about? It is called Stomatitis Virus, Ebola Virus Vaccine.
The VSV-EBOV vaccine was developed by the Public Health Agency of Canada and licensed to Merck and NewLink Genetics Corp. There were no cases of Ebola with symptom onset at least 10 days after randomization in the immediate vaccination group, compared with 16 cases in the delayed vaccination group, reflecting a vaccine efficacy of 100%.
What did the trial find?
The researchers added that even prior to the Ebola outbreak, there were certain candidates for possible vaccines but funds were inadequate to test them. Up until now, about 4,000 people have been injected All of them had had contact with the virus over the past ten days. The vaccine was therefore deemed to have provided 100% protection against the virus in this trial.
The World Health Organization (WHO) has stated that this vaccine could be “the” thing that everyone was waiting for as far as Ebola is concerned.
For the trials, half of the participants were vaccinated three weeks after identification of an infected patient. But there is already excitement about the vaccine.
On the other hand, if all eligible adults were included in the analysis, vaccine effectiveness was 75.1% and did not reach statistical significance, Kieny and colleagues reported.
The trial used an unusual strategy – “ring vaccination” – that was used in the campaign to eradicate smallpox in the 1970s. “Will it work at six months?”
Many people infected by Ebola have been injected with the vaccine that could save the lives of thousands and could help put an end to the outbreak of this deadly disease. The number of cases has fallen – and in the week up to July 26th 2015 there were just four cases in Guinea and three in Sierra Leone. The Guinean national regulatory authority and ethics review committee have approved continuation of the trial. Though, with the needed support, they would make it so that vaccines would be available for emergency use.
The trial in Guinea is the largest study ever conducted in that country, The Lancet’s editors said in an accompanying unsigned editorial, and is a “testament not only to the skill of the research teams but also to the commitment of communities to defeating an epidemic that has devastated their nation”.
It is in fact incredibly unusual for any vaccine to show such efficacy, and so swiftly, too.
Can the fast-track approach be applied to other diseases?
This causes manufactures to be less eager to invest in a vaccine’s development.
Global health experts dubbed the results as “remarkable” and “game changing”, as the study also shows the new vaccine “may help extinguish this [Ebola] outbreak”, as confirmed by Dr. Amesg Adalja, an infectious-disease specialist and a senior associate at the University of Pittsburgh Medical Center for Health Security.
Advertisement
Are lessons likely to be learned from rVSV-ZEBOV’s success? “It will change the organization of the present erupt and future flare-ups”.