-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
Hundreds of U.S. Clinics Sell Unapproved Stem Cell ‘Therapies’
While millions of haematopoietic stem cell treatments for blood-related cancers like leukaemia have been carried out for decades with proven efficacy, stem cell treatments for most other conditions are still in their embryonic research stage.
Advertisement
The online research discovered these “stem cell clinics” appear to be clustered in several states that include Florida, New York, California, Arizona, Colorado and Texas. Similar clusters were also seen in certain cites, most notably Beverly Hills, followed by NY and San Antonio. Turner and Knopfler declined to characterize the operations as illegal, instead calling for greater federal supervision of the infantile industry.
Professor Knoepfler said he is anxious about the public perception of stem cell treatments.
“In nearly every state now, people can go locally to get stem cell ‘treatments”.
The paper, published in the journal Cell Stem Cell, identified 570 clinics that offered stem cell therapies that promised to treat dozens of conditions. You can just drive down the street and get a stem cell treatment. Medical researchers have been studying the possibility of using stem cells to fix damaged tissue in a range of chronic ills – with limited success so far.
“In theory, stem cells may advance the treatment of many other diseases or conditions; however, at this time, the value of stem cells as a treatment for most conditions is largely unproven and more information is needed about their potential benefits”, the FDA said in a statement. But stem cell therapies are unexpectedly flourishing in the USA and may only require a short vehicle trip.
Researchers compiled the list by searching online, saying their results “should serve as a baseline for future studies of United States businesses engaged in direct-to-consumer advertising of purported stem cell interventions”, the report said. Ltd., Mumbai on the occasion of the 3rd annual national conference of the Stem Cell Society of India (SCSI) held at the India Habitat Center, New Delhi recently. They also argue that compelling stem cell procedures to undergo the full FDA approval process could protect the industry by ensuring its practices are safe. He talked on the limitations of conventional treatment modalities and brought into light various aspects of stem cell technology.
Michael Werner, executive director of the Alliance for Regenerative Medicine, which represents companies, patient advocates and researchers, said group thinks FDA oversight is critical.
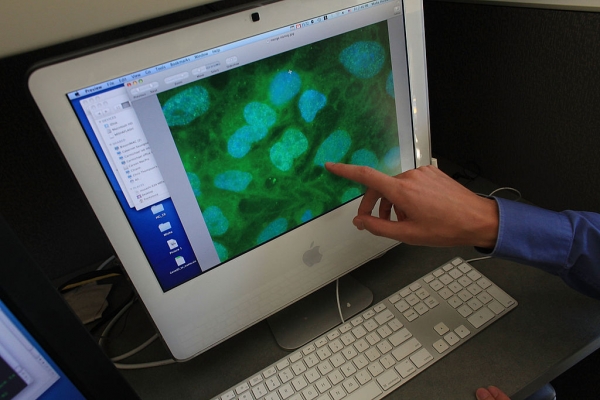
Stem cells are the starter cells for all kinds of other tissues, from blood to bone to brain, and are under intense investigation in hundreds of clinical trials as disease treatments, where the cells are usually infused or injected into sites of injury or disease.
“There is a widespread misconception that if there is a clinic in the United States… making these claims about stem-cell therapy, then that must somehow be legitimate or credible”, said Leigh Turner, a Canadian professor at the University of Minnesota’s Center for Bioethics.
From 2009 to the present, these firms have entered the market and have made routinely marketing saying they can treat between 30 and 40 stem cell disease no one has taken significant regulatory measures, said Turner.
But, if the size of the marketplace is any indication, American stem-cell clinics are doing booming business, even attracting patients from Canada, and charging thousands or tens of thousands for their services. Almost half of the clinics are offering treatments using bone marrow-based stem cell procedures. Think of them as the possible “fix-all” for diseases and mutations in the body.
“We don’t even know what causes autism”, he said.
Stem-cell clinics and their claims should be treated with extreme caution, he said. “There is an obvious need for the FDA, FTC (Federal Trade Commission), state medical boards and other regulatory bodies to play a more effective role in regulating the marketplace for stem cell interventions”, he said.
Advertisement
Turner and Knoepfler, who runs the popular stem cell blog “The Niche”, grew suspicious of an increase in American stem cell clinics when inquiries from readers and patients changed from Americans asking about going overseas for a stem cell treatment to Americans asking about seeking treatment in the United States.