-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
J&J gets FDA green light for myeloma drug Darzalex
It has also been filed for approval in Europe, where it has been granted an accelerated assessment, as well as in Canada.
Advertisement
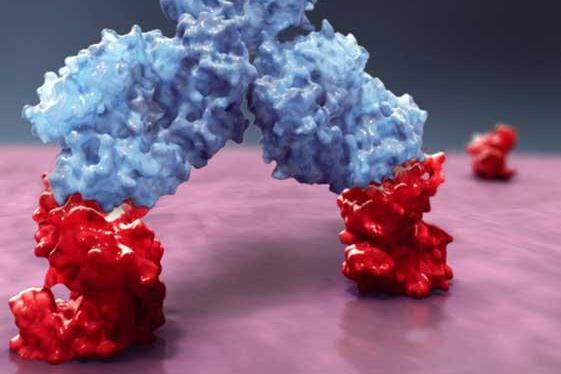
Darzalex (DAR’-zah-lecks) is the first biologic drug and first monoclonal antibody – a genetically engineered drug created to target diseased tissue and spare healthy cells nearby – approved for multiple myeloma.
Focusing on the group of patients getting the top dose, investigators reported in the New England Journal of Medicine last August that the drug scored an impressive 36% overall response rate among 42 patients with late-stage multiple myeloma-a step up from the 29% overall rate that was reported at ASCO earlier in the year. Weakened bones compromise the patient’s immune system and the patients end up having kidney problems. The National Cancer Institute estimates there will be 26,850 new cases of multiple myeloma and 11,240 related deaths in the United States this year.
Darzalex (daratumumab) is the first human anti-CD38 monoclonal antibody to be cleared anywhere in the world and will be sold by J&J’s Janssen Biotech unit for multiple myeloma patients whose cancer has become resistant to other treatments.
ORR was higher in less heavily pretreated patients, at 56% for those who received 2 or 3 prior lines of therapy compared with 23% in a more heavily pretreated population.
As for the side effects from the treatment, most patients experienced nausea, fatigue, fever, back pain and cough. Experts says Darzalex could also effect in low counts of infection-fighting white blood cells, red blood cells and low levels of blood platelets.FDA urged that blood bank must be informed when a particular patient is undergoing Darzalex dose. Because FDA is speculating that the drug would interfere and end up manipulating certain test results.FDA also alerted that women who are pregnant shouldn’t use this drug.
Women who are pregnant shouldn’t use the drug, and other women of childbearing age should use contraception for at least three months after treatment, the FDA warned.
Advertisement
As a result of the FDA approval, Genmab is now set to receive a milestone payment of $45 million that will be triggered by the first commercial sale of the product in the U.S. Priority review status is granted to applications for drugs that, if approved, would be a significant improvement in safety or effectiveness in the treatment of a serious condition.