-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
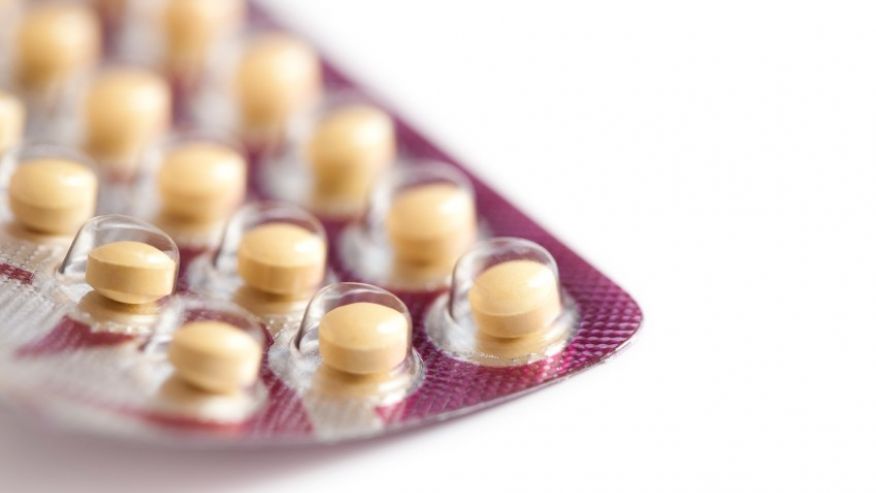
Lawsuit Alleges Birth Control Packing Error Led to Unwanted Pregnancies
More than 100 women are suing Qualitest Pharmaceuticals after they say they got pregnant while taking incorrectly packaged birth control pills. A few of them are even requesting the “total cost of raising a child until adulthood”.
Advertisement
The package was rotated 180 degrees, reversing the orientation of the pills – meaning that women took placebo pills when they should have been taking active pills.
The packaging mistake was caught back in 2011 at which time Qualitest announced a recall of eight brands of birth-control pills, but … several women were allegedly still affected by the error.
A class-action lawsuit was originally filed in Atlanta, but after a judge’s rejection, it was refiled in Pennsylvania because Endo’s USA headquarters are in the state, the report said.
A spokeswoman for Endo Pharmaceuticals said the company is aware of the complaint, but does not comment on pending or ongoing litigation. “As a result of this packaging error, the daily regimen for these oral contraceptives may be incorrect and could leave women without adequate contraception, and at risk for unintended pregnancy”.
The products in the voluntary 2011 recall included one or more versions of Cyclafem, Emoquette, Gildess, Orsythia, Previfem and Tri-Previfem.
‘The recall that forms the basis of this suit was entirely voluntary and occurred more than four years ago in September 2011. Endo Pharmaceuticals says, in part, the voluntary recall occurred based on an extremely small number of pill packs that were manufactured by an external contract manufacturer. Also, they’ve only been able to confirm one defective pack was sold to a patient.
Advertisement
We asked Philadelphia attorney Shanin Spector to weigh in on the issue, he is not directly related to the case but is no stranger to large-scale pharmaceutical settlements.