-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
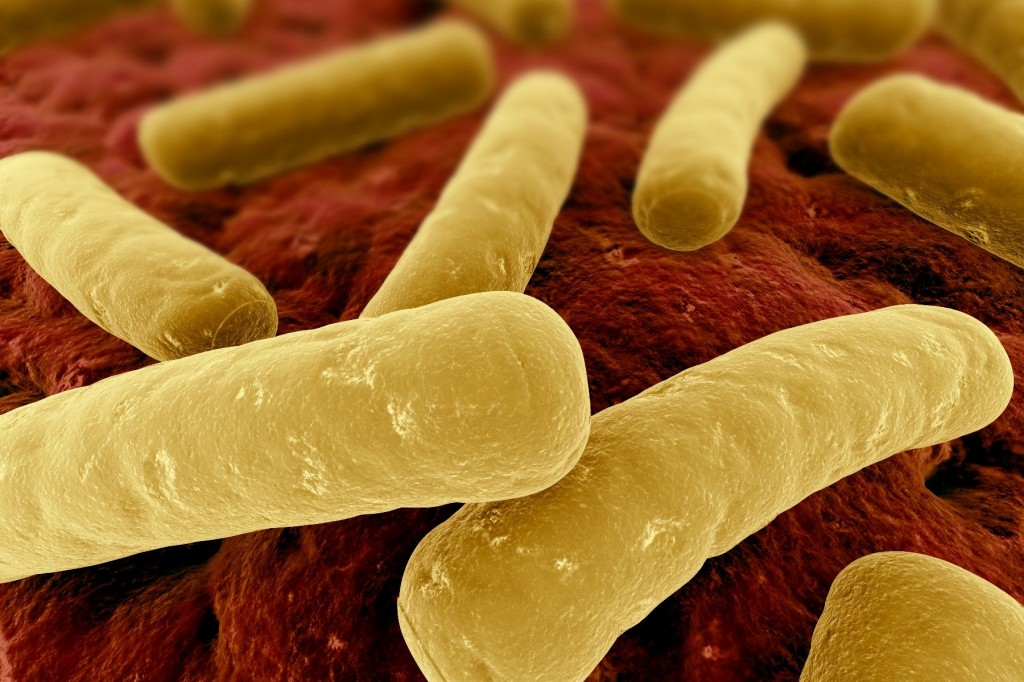
Merck’s bezlotoxumab successful in two late-stage studies for prevention of C
Bezlotoxumab was developed by researchers at the University of Massachusetts Medical School’s MassBiologics Laboratory in conjunction with Medarex (now part of Bristol-Myers Squibb), and licensed to Merck in 2009 for development as a potential therapeutic for C. difficile infection.
Advertisement
Merck further developed the drug and performed human trials. It is a notoriously recurrent infection, the incidence of which has risen sharply over the last 20 years. The an infection is handled with normal antibiotics, which additionally wipe out pleasant micro organism that usually maintain C. difficile beneath management.
The disease is one of the top causes of healthcare-acquired diseases in health care facilities; it can damage intestinal walls, trigger inflammatory processes in bowels, and lead to diarrhea that can be some times fatal. This leads to the symptoms of C. difficile, including abdominal pain and watery diarrhea. The risk of having the infection is higher in certain populations, including people of age 65 and older, or those with a compromised immune system. The recurrence among patients with placebo was 25%. Fatalities often occur within 30 days of initial diagnosis.
Merck carried out the MODIFY I study in 19 countries participated by more than 1,400 patients with a median age of 65. The studies were carried out in hospitals and outpatient settings. Typically, patients are seven to ten times more likely contract the disease when they are already antibiotics.
The research confirmed no profit from a second experimental antibody, actoxumab, both alone or together with bezlotoxumab.
Where bezlotoxumab or bezotoxumab plus actoxumab was taken, infection recurrence was significantly lower compared with the placebo.
The medical experts conducted two studies over a 12-week period to evaluate bezlotoxumab. Based on the results, The company selected bezlotoxumab for the marketing authorization application.
The broader issues of how to gain approval for antibiotics that do not conform to the classical model was discussed at a lively Saturday session on “Solving the Riddle of Developing Agents with Novel Approaches to Antimicrobial Therapy” that featured speakers from industry and US and European regulatory authorities.
Merck & Co Inc. reported data from two phase III trials of its antibody bezlotoxumab for the prevention of recurrent Clostridium difficile at the global Conference on Antimicrobials and Chemotherapy and the worldwide Congress of Chemotherapy (ICAAC/ICC) 2015 annual meeting today.
Advertisement
In MODIFY I, patients receiving standard-of-care antibiotics for C. difficile were randomized to receive a single, one-time infusion of either bezlotoxumab (10 mg/kg, n = 403), actoxumab (10 mg/kg, n = 242), the combination of bezlotoxumab and actoxumab (10 mg/kg each, n = 403), or placebo (n = 404).