-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
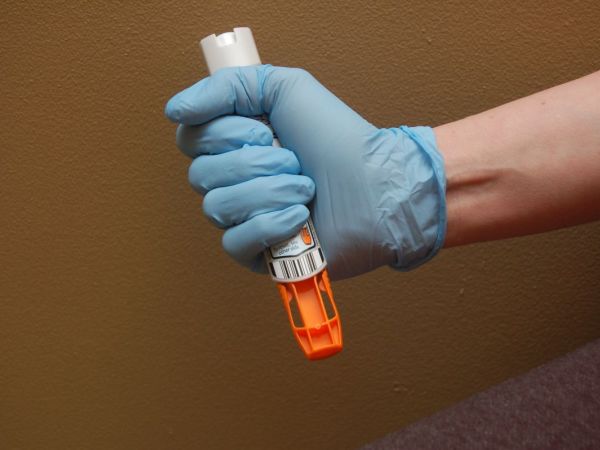
Mylan Will Launch Generic Version of EpiPen at Half the Price
In a continuation of its response to widespread criticism, pharmaceutical company Mylan NV MYL announced Monday that it will launch a generic version of the EpiPen at a 50% discount to the standard version of its popular allergy medication.
Advertisement
The drugmaker is making a second move in less than a week in response to intensifying criticism, after its offer of assistance programs to help patients cover out-of-pocket expenses was blasted as a mere press-relations fix by several U.S. lawmakers.
Identical to the original product – a pre-filled syringe containing epinephrine, used for life-threatening allergic reactions – the generic will cost $300 for two, reports the Associated Press.
The surprise move is the latest attempt by Mylan to silence the uproar ignited by a more than 400% increase in EpiPen prices. The price of the EpiPen has increased 550 percent over eight years, and the list price for a pack of two is just over $600.
Responding to an outcry over the increased price of its allergy auto-injector EpiPen, Mylan (MYL) plans to launch a cheaper generic alternative, reported Reuters.
Mylan says 80 percent of commercially insured patients paid nothing for their EpiPens previous year. It also said it will offer $300 copay cards, up from the current $100 per-prescription savings.
The company addressed why it made a decision to introduce a generic alternative that is identical to the EpiPen instead of just lowering the brand name product’s list price, according to the report.
“The weirdness of a drug company offering a generic version of its own branded but off-patent product is a signal that something is wrong”, Robert Weissman, president of consumer watchdog group Public Citizen, was quoted saying. That still will bring Mylan tens of millions of dollars while helping it retain market share against current and future brand-name and generic competition. Launching a generic version of the EpiPen can help the drugmaker protect its market share from competition.
Defending criticism on the rising prices, Bresch said in an interview with CNBC that this wasn’t a Mylan issue or an EpiPen issue but rather a health care issue.
Several members of the U.S. congress have called for a Federal Trade Commission investigation of Mylan and Food and Drug Administration measures to increase competition for the emergency allergy treatment.
Advertisement
The “complexity and opaqueness of today’s branded pharmaceutical supply chain” led Mylan to determine that “bypassing the brand system” was the “best option”, Bresch said.