-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
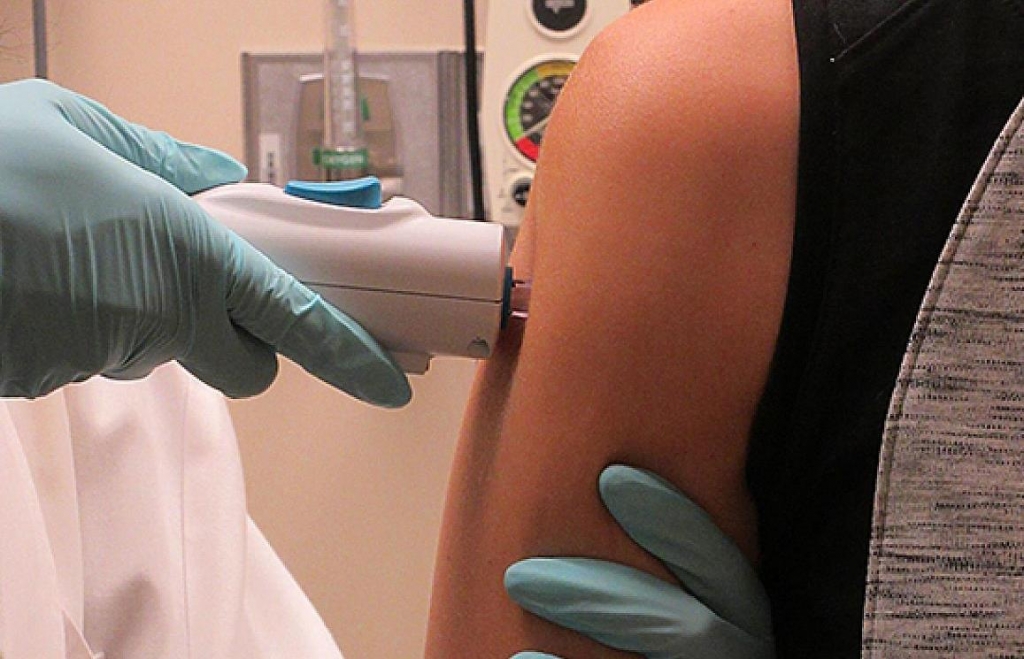
National Institutes of Health tests Zika vaccine
A pair of Aedes albopictus mosquitoes are seen during a mating ritual while the female feeds on a blood meal in a 2003 image from the Centers for Disease Control (CDC).
Advertisement
If the current set of trials have positive results, human trials will be expanded to other countries that have been affected by the Zika virus by early next year.
Zika has been shown to be responsible for an alarming increase in microcephaly, in which babies are born with small brains and heads, as well as other hearing, vision and developmental disorders.
A vaccine could roll back the epidemic from USA borders, but it won’t be known for months if the government’s vaccine is safe, and it could take years longer to prove it’s effective.
The National Institute of Allergy and Infectious Diseases (NIAID) Zika virus investigational DNA vaccine will be given to 80 healthy volunteers aged 18-35 at three study sites in the United States, said the federal agency. Anthony Fauci, the director of the National Institute of Allergy and Infectious Diseases, made the announcement, “I am pleased to report that we have begun, on August 2, testing an investigational DNA vaccine meant to prevent Zika virus infection”. The volunteers will be separated into multiple groups, so that scientists can experiment with the frequency and timing of doses.
Meanwhile, South Florida is now reporting 15 cases of local Zika transmission.
Public health officials have said small, localized outbreaks are likely in southern USA states already vulnerable to mosquito-borne disease.
White House spokesman Josh Earnest on Wednesday criticized the Republican-led US Congress for not approving the original request for Zika spending. That can happen if a pregnant woman gets Zika from a mosquito bite or through sex, as the virus also spreads between people that way. And, because the virus can be transmitted sexually by an infected partner, researchers would also be looking to prevent the disease in the general adult population.
DNA vaccines are a new focus in vaccine development, but none has been approved for use by U.S. regulators so far. The vaccine is injected into the arm muscle of a patient, where cells read the gene and start producing Zika virus proteins that self-assemble into particles reminiscent of the virus. They would be conducted in endemic countries, testing not only the safety of the Zika vaccine but its effectiveness in raising an immune response.
New York Attorney General Eric Schneiderman said his office has sent cease-and-desist letters to seven companies accused of deceptively marketing ineffective Zika-protection products. He said they were mainly “fly-by-night” operations likely to shut down and pop up elsewhere to evade law enforcement.
Advertisement
The mosquitoes in the area might be resistant to the two pesticides that have so far been used, Frieden said, or they might be breeding in hidden pools of water that have not yet been found by response teams.