-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
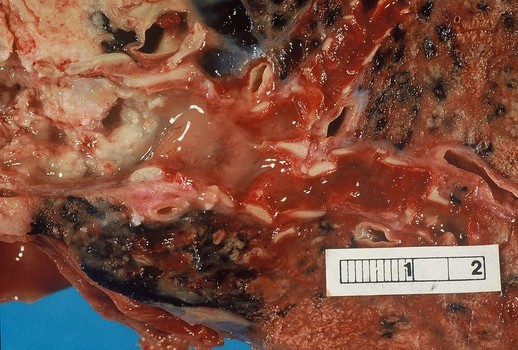
Necitumumab cleared for advanced squamous NSCLC
The FDA has approved Portrazza (necitumumab) in combination with Gemzar (gemcitabine) and cisplatin for the first-line treatment of patients with locally advanced or metastatic squamous non-small cell lung cancer (NSCLC), based findings from the phase 3 SQUIRE trial.
Advertisement
Eli Lilly and Co (NYSE:LLY) shares surged in NY pre-market trading after the U.S. Food and Drug Administration approved its drug to treat an advanced form of lung cancer.
Portrazza has been granted Orphan Drug Designation by the FDA. The recommended dose of necitumumab is 800 mg as an IV infusion over 60 minutes on days 1 and 8 of each three-week cycle.It is not indicated for the treatment of non-squamous NSCLC.
Lung cancer is the leading cause of cancer death in the US, with an estimated 221,200 new diagnoses and 158,040 deaths forecast in 2015.
The FDA ultimately sided with the committee majority and the view that approval constituted a necessary “small baby step” for a disease that has few treatment options. In the 1093-patient study, the addition of the fully human IgG1 anti-EGFR monoclonal antibody necitumumab to gemcitabine and cisplatin improved overall survival (OS) by 1.6 months, which was equivalent to a 16% reduction in the risk of death.
“We have seen advances in lung cancer in the last 20 years, but not for the initial treatment of patients battling metastatic squamous NSCLC”, said Richard Gaynor, senior vice president of product development and medical affairs for Lilly Oncology. The drug obstructs the very signal that helps the cancer tumor grow unimpeded.
“This is a complex disease and there is an urgent need for effective, first-line treatments”, he added.
A 1,093-patient clinical trial showed that patients who received Portrazza, along with chemotherapy drugs gemcitabine and cisplatin, survived an average of 11.5 months compared with 9.9 months for those who received only chemotherapy treatments.
The median OS was 11.5 months (95% CI, 10.4-12.6 months) in patients assigned to necitumumab plus GC and 9.9 months (95% CI, 8.9-11.1 months) in those assigned to GC alone (hazard ratio [HR], 0.84; 95% CI, 0.74-0.96; P = 0.01).
The most common adverse reactions observed in necitumumab-treated patients were rash and hypomagnesemia.
Advertisement
Petrazza will include a boxed warning about the risk of cardiac arrest and sudden death, as well as hypomagnesemia (magnesium deficiency).