-
Tips for becoming a good boxer - November 6, 2020
-
7 expert tips for making your hens night a memorable one - November 6, 2020
-
5 reasons to host your Christmas party on a cruise boat - November 6, 2020
-
What to do when you’re charged with a crime - November 6, 2020
-
Should you get one or multiple dogs? Here’s all you need to know - November 3, 2020
-
A Guide: How to Build Your Very Own Magic Mirror - February 14, 2019
-
Our Top Inspirational Baseball Stars - November 24, 2018
-
Five Tech Tools That Will Help You Turn Your Blog into a Business - November 24, 2018
-
How to Indulge on Vacation without Expanding Your Waist - November 9, 2018
-
5 Strategies for Businesses to Appeal to Today’s Increasingly Mobile-Crazed Customers - November 9, 2018
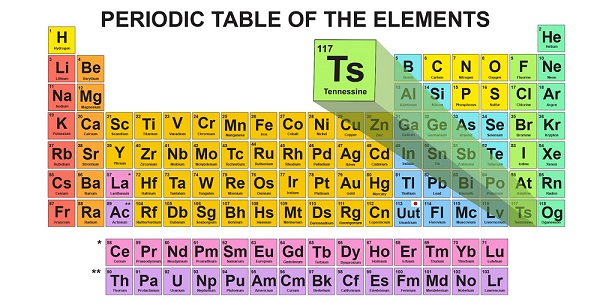
New elements named by IUPAC
The International Union of Pure and Applied Chemistry announced names for four new elements Wednesday.
Advertisement
These new elements will now be known as moscovium after Moscow, nihonium after Japan, tennessine after Tennessee and oganesson after a Russian scientists which are also recommended by an worldwide scientific group just this week.
Names have now been proposed for the four new chemical elements added to the periodic table in January. However, there are a few rules: they can only be named after a mythological figure or concept, geographical place, scientist, element property, or a mineral or similar substance. Researchers used the Dubna Gas-Filled Recoil Separator at the Joint Institute for Nuclear Research along with the heavy ion accelerator capabilities at the Flerov Laboratory of Nuclear Reactions in Moscow. Nihon is one way to say the country’s name in Japanese.
The element is scheduled to go by the initials “Ts” on the table, though the name still must undergo public review before it is formally approved. If accepted, Oganesson (Og) will only be the second element named for a living person with the first being Seaborgium, named for Glenn T Seaborg. Element 113 was discovered by scientists at RIKEN in Wako, Japan.
The proposed names of the elements are now up for public evaluation and will be confirmed in December. The name honours Russian physicist Yuri Oganessian. The names are on a five-month probation before things are made official. His many achievements include the discovery of superheavy elements and significant advances in the nuclear physics of superheavy nuclei including experimental evidence for the “island of stability”.
Still, IUPAC verified the new element and allowed its discoverers to name it. It was Hamilton who first suggested the name “tennessine”.
Advertisement
Finally, laboratories are already working on searches for the elements in the 8th row for the periodic table, and they are also working to consolidate the identification of copernicium and heavier elements. They’re synthetic elements that can only be created in the lab, and they decay so fast after synthesis, for years the teams behind them didn’t have a chance to get a proper look before they morphed into something else entirely.